P139 Prevalence of infections in patients affected by inflammatory bowel disease treated with anti-TNF-α agents: a single-centre experience
N. Imperatore1, M. Foggia2, A. Rispo1, M. Patturelli1, G. Calabrese2, A. Testa1, O. Nardone1, L. Pellegrini1, A.D. Guarino1, S. Ricciolino1, G. Tosone2, F. Castiglione1
1Federico II University of Naples, Department of Clinical Medicine- Gastroenterology and Surgery, Naples, Italy, 2Infective Diseases Unit- School of Medicine Federico II of Naples, Department of Clinical Medicine and Surgery, Naples, Italy
Background
Although the efficacy of anti-TNF α agents has dramatically changed the current management of inflammatory bowel disease (IBD), their safety represents an important issue in prescribing anti-TNF. In particular, anti-TNF α treatment has been associated with higher risk of developing infective disease, such as tuberculosis (TBC) and cytomegalovirus (CMV) reactivation and other viral/bacterial diseases. The aim of the present study was to evaluate the incidence and prevalence of CMV, TBC, hepatitis B (HBV) and C (HCV) infection/reactivation and other infections in IBD subjects treated with anti-TNF α.
Methods
retrospective analysis of prospective maintained database including all IBD subjects treated with anti-TNF α (infliximab, adalimumab and golimumab) for at least 1 year in the period 2013–2018, whose infective serological status (Quantiferon TB test, Mantoux, CMV IgM/IgG, HBsAg, HBsAb, HBcAb, HBeAg, HBeAb, anti-HCV, anti-HIV, HSV IgM/IgG, VZV IgM/IgG, EBV IgM/IgG) was known before starting the treatment and during the follow-up. Incidence (number of infections per 100 patient-years) and prevalence of each infection was reported.
Results
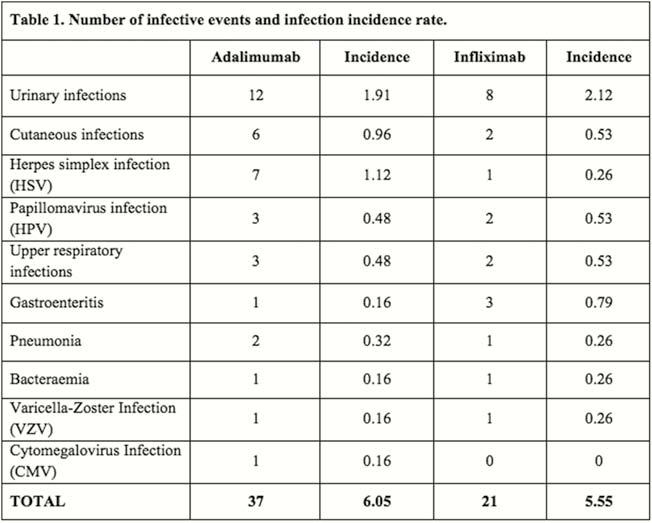
Among 689 who started an anti-TNF α agent, 288 subjects (males 52.8%, mean age 28.5 + 12.2 years, Crohn’s disease 82.3%), met inclusion criteria and were enrolled. Total years/patient were 378.08 for infliximab, 627.58 for adalimumab and 8.25 for golimumab. Before starting treatment, CMV IgG antibodies were detectable in the majority (78.8%) of patients, but no case of IgM or CMV-DNA positivity was recorded; three subjects (1%) had latent TBC infection (LTBI) and were treated with isoniazide before starting anti-TNF; one case of HBV and one case of HCV infection were registered. During the anti-TNF α treatment, a total of 58 infective events (20.1%) were recorded: 63.8% during adalimumab and 36.2% during infliximab treatment. The most common infections were: urinary (34.5%), cutaneous (13.8%), HSV (13.8%), HPV (8.6%), upper respiratory infections (8.6%), gastroenteritis (6.9%), pneumonia (5.2%), bacteraemia (3.4%), VZV (3.4%) and de novo CMV (1.7%). Among them, 13 (22.4%) were considered severe, 11 (19%) needed hospitalisation and 9 (15.5%) led to anti-TNF withdrawal. No case of CMV or TBC reactivation was registered during the follow-up. The infection incidence rate was therefore 6.05/100 patient-years for adalimumab and 5.55/100 patient-years for infliximab (
Conclusion
IBD patients are at high risk of developing infective disease during anti-TNF α therapy. The recognition of pre-exposure serological status, as well patients’ strict monitoring during maintenance treatment, dramatically reduces the risk of severe reactivation (in particular TBC and CMV reactivation).


