P146 Comparison of three histologic indices in ulcerative colitis: Evaluation from the Phase 2 study of upadacitinib (U-ACHIEVE)
V. Jairath1, W.J. Lee2, W. Zhou3, G. Heap3, J. Butler4, A.L. Shields5, S. Ollis5, N. Harpaz6
1Western University, Department of Medicine and Department of Epidemiology and Biostatistics, London, Canada, 2AbbVie Inc., Health Economics and Outcomes Research, North Chicago, USA, 3AbbVie Inc., Immunology, North Chicago, USA, 4AbbVie Inc., Clinical Development, North Chicago, USA, 5Adelphi Values, Patient Centered Outcomes, Boston, USA, 6Icahn School of Medicine at Mount Sinai, Division of Hematology and Oncology- Department of Medicine and Department of Pathology, New York, USA
Background
Several histologic indices are available for evaluating mucosal inflammation activity in ulcerative colitis (UC), although there is no consensus regarding which is the most appropriate for evaluating disease activity in clinical trials.
Methods
The correlation of three histologic indices, the Geboes Score (GS, range of major grade 0 to 5), Robarts Histopathology Index (RHI, range 0 to 33), and Nancy Index (NI, range grade 0 to grade 4) were evaluated in a Phase 2b, multicenter, randomised, double-blind, placebo-controlled study of upadacitinib in patients with moderately to severely active UC. Biopsy data were collected and centrally read using GS at Baseline and week 8; scores of RHI1 and NI2 were derived based on GS. Descriptive statistics, correlational analyses, and stratified analyses were performed in the pooled study population using as observed data.
Results
Among 250 UC patients enrolled at Baseline, 224, 214, 209 patients with non-missing values of GS, RHI and NI were observed, respectively. At week 8, the GS, RHI, and NI correlated strongly to each other (
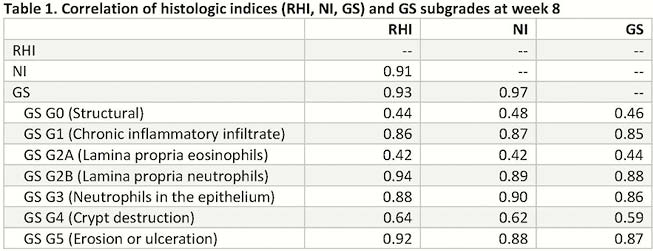
Conclusion
In a moderately to severely active UC population, GS, RHI and NI correlate strongly with each other. Higher correlations were found between histologic scores and endoscopic activity than symptom-related disease activity. Further studies are warranted to evaluate the relationship between histological inflammation and long-term clinical outcomes.
Mosli MH, et al. Gut 2017;66:50–58 2. Colombel JF, et al. Gut 2017;66:2063–2068
Acknowledgements and Funding statement: Design, study conduct, and financial support for the study were provided by AbbVie; Financial support for the study was provided by AbbVie. AbbVie participated in interpretation of data, review, and approval of the abstract. All authors contributed to development of the abstract and maintained control over final content.


