P151 Assessing comprehensive remission for Ulcerative Colitis in clinical practice: International consensus recommendations
Schreiber, S.W.(1)*;Danese , S.(2);Dignass, A.(3);Domènech , E.(4);Fantini , M.(5);Ferrante, M.(6);Halfvarson, J.(7);Hart, A.(8);Magro, F.(9);Lees, C.(10);Leone, S.(11);Pierik, M.(12);Field, P.(13);Schofield, H.(13);Peyrin-Biroulet , L.(14);
(1)Kiel University/UKSH, Clinic of Internal Medicine I, Kiel, Germany;(2)IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Gastroenterology and Gastrointestinal Endoscopy Unit, Milan, Italy;(3)Agaplesion Markus Hospital- Goethe University, Department of Medicine I, Frankfurt, Germany;(4)Hospital Universitari Germans Trias i Pujol and CIBEREHD, Department of Gastroenterology, Barcelona, Spain;(5)University of Cagliari, Department of Medical Science and Public Health, Cagliari, Italy;(6)University Hospitals Leuven- KU Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(7)Faculty of Medicine and Health- Örebro University, Department of Gastroenterology, Örebro, Sweden;(8)St. Mark's Hospital, Faculty of Medicine- Department of Metabolism- Digestion and Reproduction, London, United Kingdom;(9)Centro Hospitalar Universitário de São João, Department of Clinical Pharmacology, Porto, Portugal;(10)Western General Hospital, Edinburgh inflammatory bowel disease unit, Edinburgh, United Kingdom;(11)European Federation of Crohn's & Ulcerative Colitis Associations EFCCA, Italian IBD Patient Association AMICI Onlus, Brussels, Belgium;(12)Maastricht University Medical Centre, Division Gastroenterology and Hepatology, Maastricht, The Netherlands;(13)Oxford PharmaGenesis, VDP Oxford Central, Oxford, United Kingdom;(14)Nancy University Hospital- University of Lorraine, Gastroenterology Department, Lorraine, France;
Background
Real-world treatment of ulcerative colitis (UC) requires a patient-centric, holistic definition of ‘comprehensive remission' that goes beyond standard regulatory definitions. In an international initiative, we reviewed aspects of UC that affect patients and attempted to achieve consensus on which aspects should be considered in a definition of ‘comprehensive remission’, using a modified Delphi process. This initiative summarized expert consensus recommendations on aspects of UC that are important to patients and that affect their lives, how to measure and score these aspects, and objective markers of mucosal inflammation that are relevant to evaluate in remission, such as endoscopic and histological activity and inflammatory biomarkers.
Methods
The Delphi consensus panel comprised 10 physicians with expertise in UC and one patient advocate, and two additional physicians chaired the process. To inform the Delphi statements, we asked 18 patients about their experiences of living with UC in remission, asked panel members about their experience of treating patients and systematically reviewed associations between outcome measures in UC. Panel members voted on a 6-point Likert scale in three rounds of anonymous voting, the final round of which was preceded by a live discussion. Consensus was met if ≥ 67% of the panel agreed with the statement (4–6 on the 6-point Likert scale). Statements not meeting consensus in voting rounds 1 and 2 were revised and any not meeting consensus by the end of voting round 3 were discarded (Figure 1). 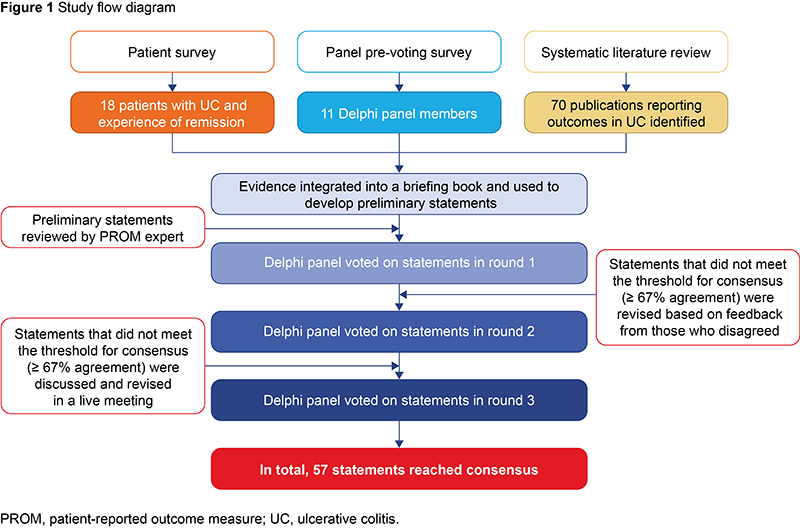
Results
The panel agreed that a definition of comprehensive remission should include aspects of UC that are currently measured in clinical trials (blood in stools, stool frequency, disease-related quality of life [QoL], endoscopic remission, histological inflammatory activity, inflammatory biomarkers and discontinuation of corticosteroids), but also additional patient-reported symptoms should be added. These were bowel urgency, abdominal pain, extra-intestinal manifestations, fatigue and sleep disturbance (Table 1). Suggestions for how the severity could be measured and thresholds for remission were agreed for each aspect, with the exception of extra-intestinal manifestations. Disease activity evaluated using ultrasound was voted as important to consider but was not recommended to be included in a measure of comprehensive remission at this stage.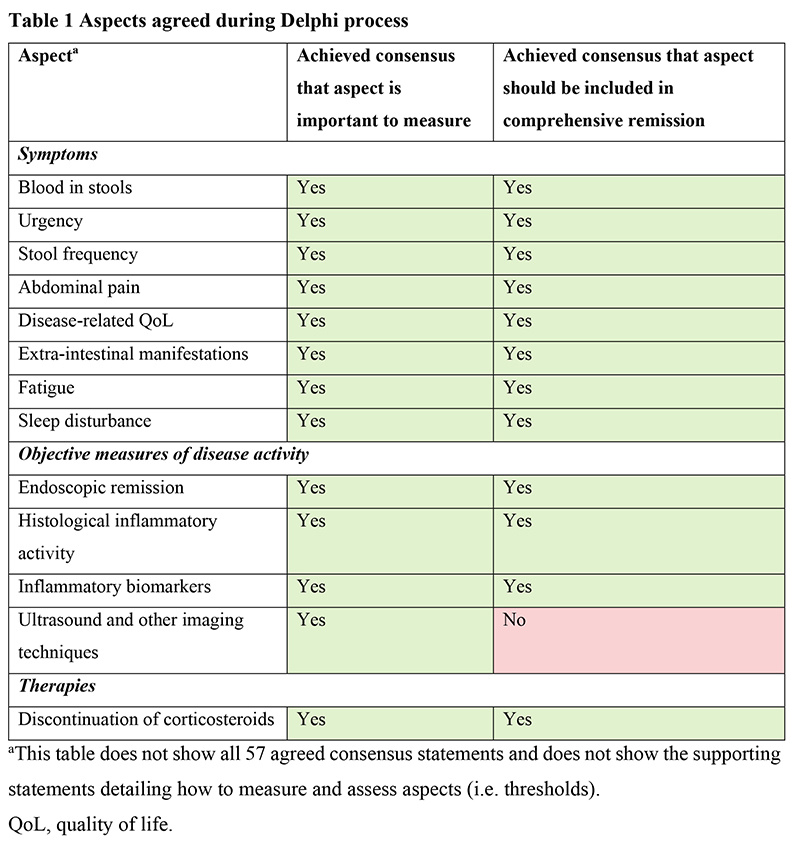
Conclusion
We used a robust methodology to reach agreement and provide recommendations on the aspects of UC that can be used to define comprehensive remission and assessed in clinical practice. We plan to develop this as a combined measure which may also have applicability in clinical trials.


