P151 Can two different faecal calprotectin assays be used interchangeably in inflammatory bowel disease treatment?
E. Van Wassenaer MD1, K. Diederen1, A. Kindermann1, E.M.M. van Leeuwen2, G.R. D’Haens3, M.A. Benninga1, B.G.P. Koot1
1Emma Children’s Hospital- Amsterdam UMC- University of Amsterdam, Pediatric Gastroenterology, Amsterdam, The Netherlands, 2Amsterdam UMC- University of Amsterdam, Department of Experimental Immunology, Amsterdam, The Netherlands, 3Amsterdam UMC- University of Amsterdam, Gastroenterology and Hepatology, Amsterdam, The Netherlands
Background
Faecal calprotectin (FC) is a broadly used sensitive biomarker for intestinal inflammation in inflammatory bowel disease (IBD) patients. However, in practice, interpretation of results can be complicated due to the use of different assays to determine FC. There is a lack of data comparing different FC assays. This study aimed to assess the agreement between two different assays for determining FC in patients with IBD.
Methods
Between January and April 2017, faecal samples of known IBD patients were tested with two assays: (1)
Results
A total of 171 patients (mean age 33 years (range: 7–81), 92 (54%) female, 117 (68%) Crohn’s disease, 53 (31%) ulcerative colitis, 1 (1%) IBD-U) were included in this study. Median FC levels were 242 mg/kg (range: 4−1800), and the mean difference between the two assays was 89 mg/kg (range: -1341 – 1140). The mean difference was largest in the high FC category, (3: 200 mg/kg,
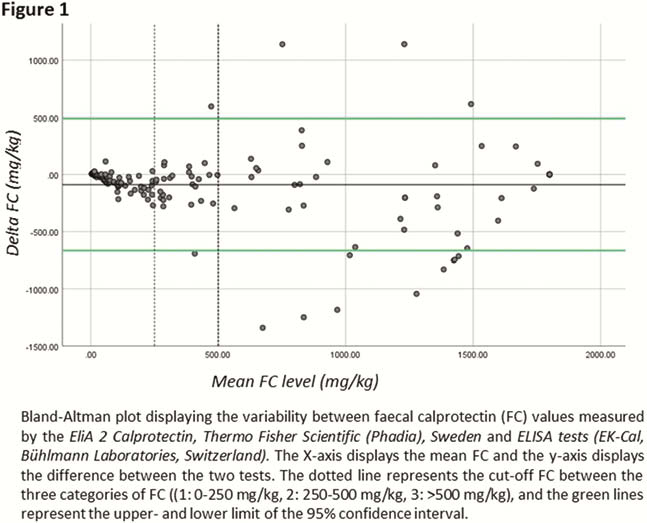
Conclusion
There is no systematic difference in FC measurements between the


