P183 A systematic review on outcome measures of long-term efficacy in clinical trials concerning Crohn’s disease patients
Janssen, L.(1,2);Creemers, R.(1,3);van Bodegraven, A.(3);Pierik, M.(1,2);
(1)Maastricht University Medical Centre+, Department of Internal Medicine- Division of Gastroenterology and Hepatology, Maastricht, The Netherlands;(2)Maastricht University Medical Centre+, School for Nutrition and Translational Research in Metabolism NUTRIM, Maastricht, The Netherlands;(3)Zuyderland Medical Centre, Department of Gastroenterology- Geriatrics- Internal and Intensive Care Medicine, Heerlen-Sittard-Geleen, The Netherlands;
Background
Treatment goals for Crohn’s disease (CD) tend to shift from clinical towards endoscopic remission as a potential means to reduce intestinal damage. Additionally, sustained corticosteroid (CS) free remission is warranted to prevent drug related side effects.
In clinical trials, long-term efficacy of treatment is assessed by a range of outcome measures at variable time points. Besides clinical activity, it is recommended by the EMA to measure biochemical and endoscopic activity, given the poor correlation between commonly applied clinical activity indices and mucosal inflammation. Finally, STRIDE advices to add a PROM given the frequent perception gap in disease control between patient and health care professionals.
Moreover, the relapsing-remitting nature of CD challenges timing of efficacy evaluations. Use of cross-sectional outcomes only on predetermined moments disregards the health status in between these measurements.
In this systematic review, we provide an overview of the outcomes employed to assess long-term efficacy of maintenance (drug) therapy or treatment strategies in randomized controlled trials and their open label extensions in CD patients.
Methods
A systematic search of Pubmed and Embase databases was performed. Giving the emerging treatment goals, the search was restricted to trials published since 2007.
Two independent reviewers screened titles and abstracts and selected eligible trials. We assessed whether and when long-term (i.e. >48 weeks) clinical, biochemical and/or endoscopic efficacy outcomes were used. In addition, data on patient perspective and CS use were collected.
Results
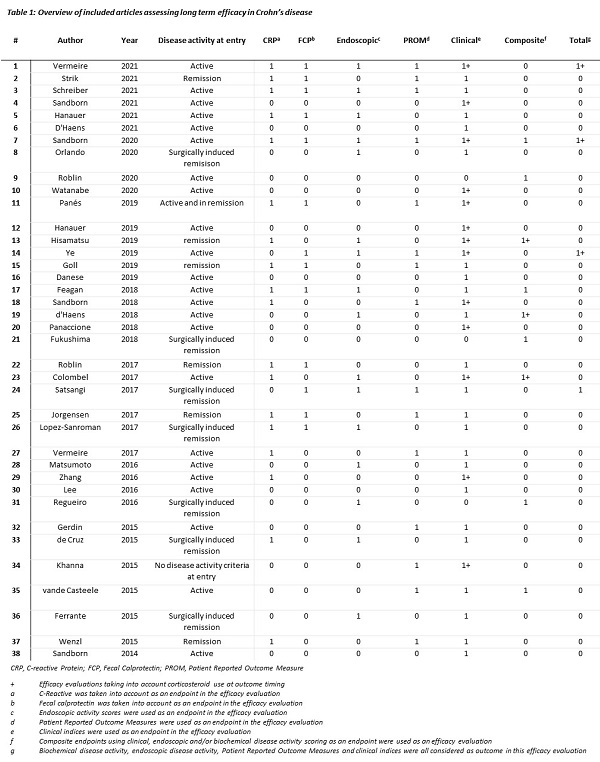
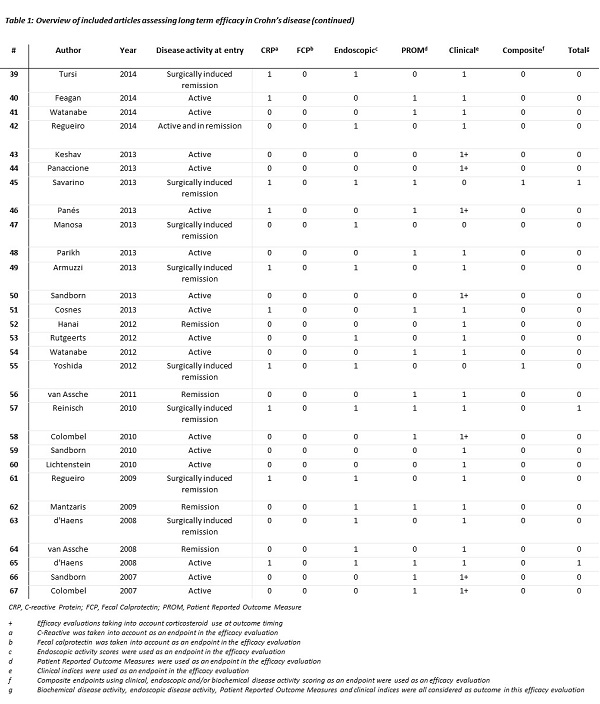
Sixty-seven out of 1947 articles were included (figure 1).
Clinical activity indices were used in 66 studies (99%) as long-term efficacy outcome, with 20 studies (30%) taking into account concomitant CS use. C-reactive protein concentration was (part of) an outcome in 29 studies (43%), and faecal calprotectin in 15 studies (22%). Endoscopic activity was evaluated in 30 studies (45%). PROMs were used in 29 studies (43%), with IBDQ being the most frequently used. Seven studies measure long-term clinical, biochemical, endoscopic activity and patient perspective, in three studies a sustained CS free status as part of the outcome was required (table 1). In most studies cross-sectional measures or multiple measurements over time, so-called panel data, were used.
Conclusion
We established a lack of objective outcome measures of inflammation and patient perspective in clinical trials in CD, consequently missing pivotal data to evaluate current treatment goals. Additionally, cross-sectional outcomes on fixed time points were mostly applied, resulting in loss of efficacy information in chronic relapsing-remitting diseases.