P193 Validation of the two-item UC patient-reported outcome (PRO2) against the simple clinical colitis activity index (SCCAI), measures of biochemical disease activity and quality of life
M. Samaan1, G. Cunningham1, G. Tamilarasan1, S. Ray1, J. Mawdsley1, S. Anderson1, J. Sanderson1, Z. Arkir2, P. Irving1
1Departmeng of Gastroenterology, Guy’s and St Thomas’ NHS Foundation Trust, London, UK, 2Viapath Laboratories, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Background
Patient-reported outcomes (PRO) have an increasingly important role in IBD. This is true of monitoring of disease activity in clinical practice and as well as the evaluation of new therapies, when used as clinical trial endpoints. An interim two-item PRO (PRO2) has been developed for UC that ranges from 0–6 and consist of the patient-derived items from the Mayo score (rectal bleeding and stool frequency). PRO2 was internally validated against endoscopic outcomes. We aimed to add external validation across a range of clinical, biochemical and quality of life outcomes.
Methods
Data were collected as part of the GO-LEVEL study, an open-label, phase IV, investigator-initiated study (NCT03124121) which included a prospective cohort of UC patients commencing golimumab induction therapy, as well as a cross-sectional cohort receiving maintenance treatment. Patients commencing induction therapy all had disease activity objectively confirmed and were evaluated at weeks 6, 10 and 14. Patients receiving maintenance therapy were recruited either at the point of flare or during stable remission. Clinical disease activity was evaluated using PRO2 and SCCAI, quality of life using IBD-Control as well as a 100mm visual analogue scale (IBD-Control-VAS), and biochemical activity using FC and CRP. Clinical remission was defined as a score of <3 using SCCAI, or 0 using PRO2. Correlations were calculated using Spearman’s correlation coefficient (rs) and contingency table analyses using Chi-square.
Results
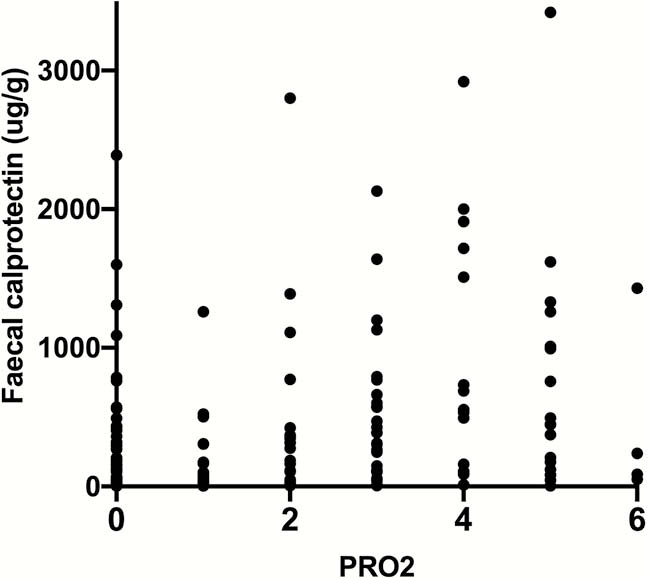
GO-LEVEL included a total of 112 patients across the two study cohorts. A total of 217 PRO2 assessments were made with concurrent SCCAI and IBD-Control scores available at all timepoints. CRP measurements were available at 214 of these and FC at 207. Strong correlations were observed between PRO2 and SCCAI (
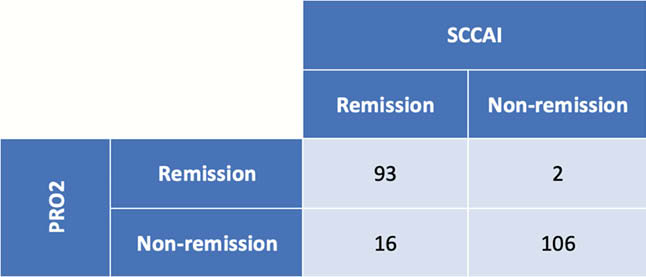
Of the 217 assessments, 109 were in SCCAI defined remission, 95 were in PRO2 defined remission and the two were highly contingent (

Conclusion
The PRO2 performs well when validated against an established clinical disease activity index (SCCAI), quality-of-life assessments and biochemical markers of disease activity. PRO2 assessments have the benefit of being more rapid to administer (comprising of only two items) than SCCAI (6 items), whilst providing similar and accurate evaluations of remission status.


