P195 Cost-effectiveness of a 17-gene classifier to guide initial treatment choice in Crohn’s disease in the UK
V. Buchanan1, S. Griffin1, J. Lee2, E. Mckinney2, P. Kinnon3, K. HILLS3
1Cogentia Healthcare Consulting, n/a, Cambridge, UK, 2Department of Medicine, University of Cambridge, Cambridge, UK, 3Department of Medical Affairs, PredictImmune Limited, Cambridge, UK
Background
PredictSURE IBD™ is a CE-marked whole blood-based biomarker test that predicts long-term clinical outcomes in inflammatory bowel disease (Crohn’s disease, CD and ulcerative colitis, UC). PredictSURE IBD™ uses a 17-gene qPCR-based classifier to stratify patients into two prognostic subgroups, high and low risk. High-risk patients experience significantly more aggressive disease than low-risk patients, with the need for earlier and more frequent treatment escalation over time. Early stratification could enable personalised treatment strategies, such as ‘top-down’ use of biologics in high-risk patients. Our objective was to examine the cost-effectiveness of PredictSURE IBD™ in guiding the use of early biologic therapy in newly diagnosed CD patients in the UK.
Methods
A decision tree leading into a Markov state-transition model was constructed in MS Excel to compare two treatment approaches: (1) standard of care therapy following established UK clinical guidelines, consisting of sequences of immunomodulator followed by biologic upon relapse (‘step-up’ treatment), (2) targeted therapy guided by PredictSURE IBD™, whereby patients identified as high-risk receive sequences of anti-TNF biologic treatment followed by other biologic classes upon relapse (‘top-down’ treatment), Figure 1. Parameters were informed by patient data from PredictSURE IBD™ clinical studies and the literature.
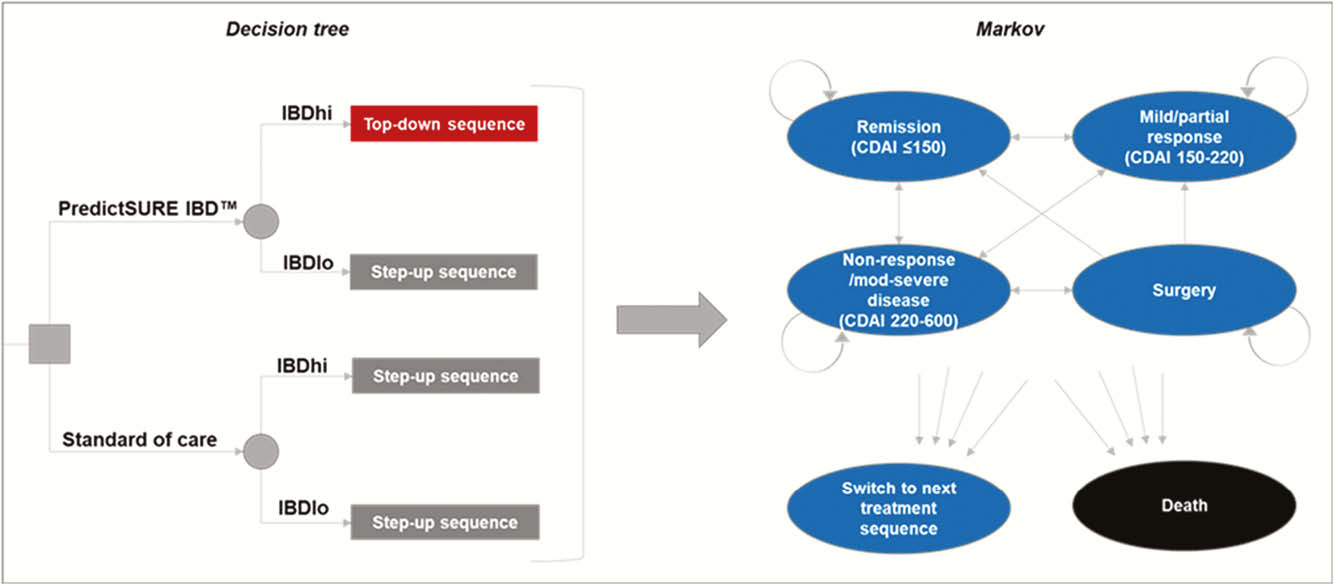
Results
Top-down treatment guided by PredictSURE IBD™ resulted in an incremental cost-effectiveness ratio (ICER) of £7,179 per quality-adjusted life-year (QALY), with £1,852 incremental costs and 0.258 incremental QALYs vs. standard of care generated over a 15-year time horizon. Additional costs relating to earlier biologic use were offset by reductions in the costs of flares, hospitalisations and surgery. Incremental QALYs were driven by increased time spent in remission and improved quality of life from reduced flares and surgery. The model was most sensitive to the time horizon, rates of mucosal healing on top-down vs. step-up therapy, the costs of hospitalisation and the costs and quality of life in the severe disease health state.
Conclusion
Modelling shows that upfront use of biologic guided by PredictSURE IBD™ could substantially improve clinical outcomes for high-risk patients by increasing remission rates and reducing flares, surgery and treatment escalations. The ICER for PredictSURE IBD™ was well below the £20–£30k/QALY threshold used by the UK National Institute for Health and Care Excellence (NICE). Top-down treatment guided by PredictSURE IBD™ would not only represent a treatment paradigm shift for CD patients but would also be a highly cost-effective use of resources in the UK National Health Service.


