P220 Predictors of prognosis after discontinuation of first-use biologics in IBD patients with at least 24 months follow-up
U.N. Shivaji1, A. Bazarova2, T. Critchlow3, S.C. Smith4, O.M. Nardone4, M. Love3, J. Davis3, S. Ghosh1, M. Iacucci1
1Department of Gastroenterology, Birmingham, NIHR Birmingham Biomedical Research Centre, UK, 2Institute for Biological Physics, University of Cologne, Cologne, Germany, 3Department of Gastroenterology, University Hospitals Birmingham, Birmingham, UK, 4Institute of Immunology and Immunotherapy, University of Birmingham, Gastroenterology, Birmingham, UK
Background
In clinical practice, patients with IBD have their biologic therapies withdrawn due to variety of reasons. The aim of the study was to report on predictors of prognosis in IBD patients after biologics have been discontinued, with a minimum follow-up of 24 months.
Methods
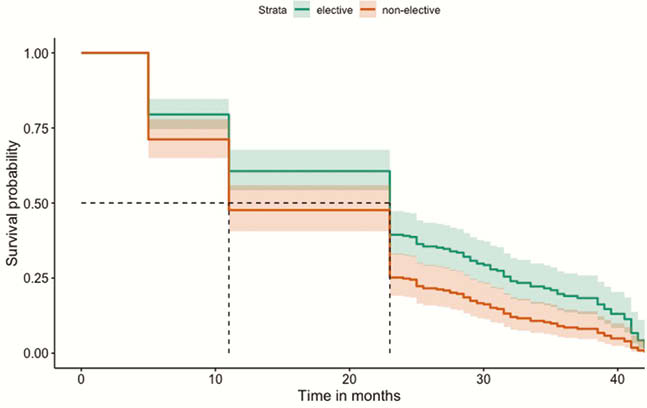
All IBD patients who discontinued their first-use biologic were identified between January 2013 and Dec 2016 from EMR at a tertiary referral centre, to ensure at least 24 months follow-up. Reasons for discontinuation and pre-defined adverse outcomes (steroid and other rescue therapies, hospitalisations, surgery including perianal) were recorded. The data were analysed using multivariable and univariable logistic regressions within a machine learning technique in order to predict adverse outcomes, within the stated timeframe. We tested the significance of the identified predictors and performed Kaplan–Meier survival analysis to compare patients with elective vs. non-elective discontinuation of biologics.
Results
147 patients who discontinued biologics (M = 74, median age 39y; CD = 110) were identified. Follow-up ranged from 24 to 60 months (median 40 months). The reasons for non-elective discontinuation included side effects (
Using data from all 147 patients, multivariable logistic regression analysis was done to identify significant predictors of prognosis. These are represented in Table 1.
Overall, a significant number of patients (
| Predictors of poor outcomes | Time interval when significant | AUROC | Odds ratio (OR) and confidence intervals | Statistically significant? |
| Asian race | < 6 months | 0.6283 | OR 0.68 95% CI (0.49,0.95) | Yes |
| Secondary non-response | < 6 months6–12 months12–24 months>24 months | 0.6715 | OR 7.09 95% CI (1.60,29.97) | Yes |
| Steroid therapy | 12–24 months | 0.6219 | OR 0.24 95% CI (0.04,0.88) | Yes |
| Predictors of good outcomes | ||||
| Male sex | >24 months | 0.638 | OR 0.31 95% CI (0.09,0.85) | Yes |
| Elective stop | <6 months6–12 months | 0.6398 | OR 0.24 95% CI (0.05,0.77) | Yes |
Conclusion
Among IBD patients who discontinued biologics, there were fewer AO when they were electively discontinued. However, majority of patients required restart of biologics to manage their disease during the follow-up period. Clinicians need to be cautious when considering biologic discontinuation given the high proportion of AO and re-escalation to biologics therapy.


