P244 Clinician preferences in treating steroid resistant ulcerative colitis: a discrete-choice experiment (DCE) survey
Wickramasekera, N.(1);Shackley, P.(1);Coates, E.(1);Barr, A.(1);Lee, M.(2);Blackwell, S.(3);Bedford, H.(3);Dames, N.(3);Probert, C.(4);Sebastian, S.(5);Lobo, A.(2);
(1)University of Sheffield, School of health and related research, Sheffield, United Kingdom;(2)Sheffield Teaching Hospitals NHS Foundation Trust, Gastroenterology, Sheffield, United Kingdom;(3)Patient representative, Patient representative, Patient representative, United Kingdom;(4)University of Liverpool, Department of Molecular and Clinical Cancer Medicine, Liverpool, United Kingdom;(5)Hull University Teaching Hospitals NHS Foundation Trust, Gastroenterology, Hull, United Kingdom
Background
The optimum treatment for steroid resistant ulcerative colitis (SRUC) is not clear and a range of options can be considered. The aim of this study was to quantify the relative importance of different treatment characteristics to clinicians and understand their preferences for benefit-risk trade-offs.
Methods
A discrete choice experiment (DCE) was conducted in the UK via online survey of clinicians with expertise in inflammatory bowel disease. This involved 12 tasks where respondents selected a preferred treatment option when presented with two competing, hypothetical treatment profiles for a SRUC scenario. Profiles described five treatment characteristics focusing on clinical outcomes and safety, identified from qualitative interviews with clinicians and evidence from systematic reviews. DCE responses were analysed using conditional logistic regressions. Regression coefficients were used to calculate benefit-risk trade-offs, to find the rate at which clinicians are willing to trade levels of risks in exchange for the preferred levels of clinical outcomes. Regression coefficients were also used to predict uptake rates for selected drugs currently prescribed to patients with SRUC.
Results
116 clinicians completed the survey (age 46y; female 42%; consultant 60%; nurse 26%; mean experience 11y). Figure 1 shows the treatment characteristics that make respondents more likely (positive coefficient) or less likely (negative coefficient) to choose a treatment. One unit increase in both lymphoma risk and serious infection risk were strongly considered when clinicians were choosing a treatment compared to one unit increase in the symptom improvement characteristics. Clinicians would accept a higher lymphoma risk of 5 per 10,000 patient years for a 10% improvement in remission rate compared to 4 and 2 cases per 10,000 patient years for 10% better rates of clinical response and mucosal healing respectively. Clinicians at secondary hospitals accepted a lymphoma risk of 4 cases per 10,000 patient years for a 10% improvement in remission vs 7 per 10,000 patient years at tertiary centres. Similar trade-off results were found for serious infection risks. Predicted uptake comparing preferred characteristics to existing treatments was infliximab (62%), tofacitinib (18%), vedolizumab (15%) and adalimumab (5%).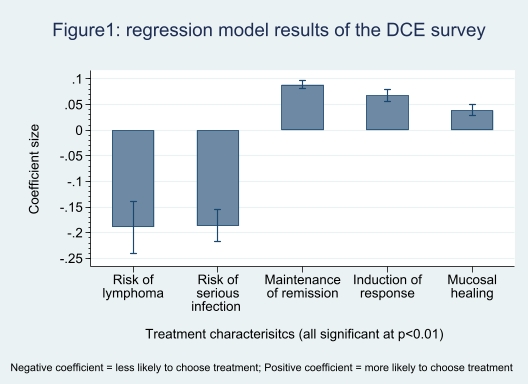
Conclusion
Clinicians are willing to make difficult trade-offs and tolerate elevated safety risks in exchange for therapeutic benefits. Risk tolerance was highest if the treatment improved long term remission rate, lowest for mucosal healing and higher in clinicians at tertiary centres. The results help to better understand treatment decisions and identify outcomes that might be considered in choosing agents to evaluate in clinical trials.


