P246 Development of a new Inflammatory Bowel Disease Patient Identifier shortens time to clinic review and initiation of treatment
Chee, D.(1,2);Hamilton, B.(1,2);Cairnes, V.(2);Lin, S.(1,2);Chanchlani, N.(1,2);Ahmad, T.(1,2);Goodhand, J.(1,2);Kennedy, N.(1,2);
(1)IBD Pharmacogenetics Group, University of Exeter, Exeter, United Kingdom;(2)Royal Devon and Exeter NHS, Department of Gastroenterology, Exeter, United Kingdom
Background
Timely diagnosis of inflammatory bowel disease (IBD) is important because earlier use of biologic therapies leads to mucosal healing, a reduction in hospitalisations and surgeries and improvements in quality of life. In the United Kingdom, general practitioners refer patients with symptoms suggestive of IBD to colorectal surgeons, emergency department physicians, gastroenterologists or directly for a lower gastrointestinal endoscopy. Consequently, there is variation in the time from endoscopic diagnosis of IBD to specialist review. We created an electronic tool that screens all endoscopy reports for a new diagnosis of IBD. These cases are then reviewed in our weekly complex multidisciplinary team (MDT) meeting and new patients are allocated an IBD specialist. We sought to determine whether our new patient identifier reduced the time to clinical review by an IBD specialist and initiation of IBD treatment.
Methods
We designed a retrospective observational cohort study comparing time from endoscopic diagnosis of IBD to initiation of treatment and IBD specialist review. Outcomes were compared in the 18 months before and after the introduction, in January 2018, of our new IBD patient identifier. Demographic, endoscopic and treatment outcome data were recorded from our electronic patient record. Categorical and continuous variables were summarised as frequencies (%) and median [IQR] and compared with Fisher’s exact and Mann Whitney U tests respectively.
Results
Between the 1st January 2018 and 30th June 2019, 116 new patients diagnosed with IBD were identified and reviewed in our MDT meeting. In the preceding 18 months, 111 patients were diagnosed with IBD. There were no significant differences between groups according to sex, age, age at diagnosis, disease type, or phenotype according to the Montreal classification (Table 1). Both the median time from endoscopic diagnosis to treatment initiation (0 days [IQR 0 – 14.2] vs 14 days [IQR 0 – 31.6], p=0.007) and specialist clinic review (21 days [IQR 7 - 25] vs 48 days [IQR 34 – 63], p<0.0001) were shorter following the introduction of the new IBD patient identifier screening tool (Figure 1).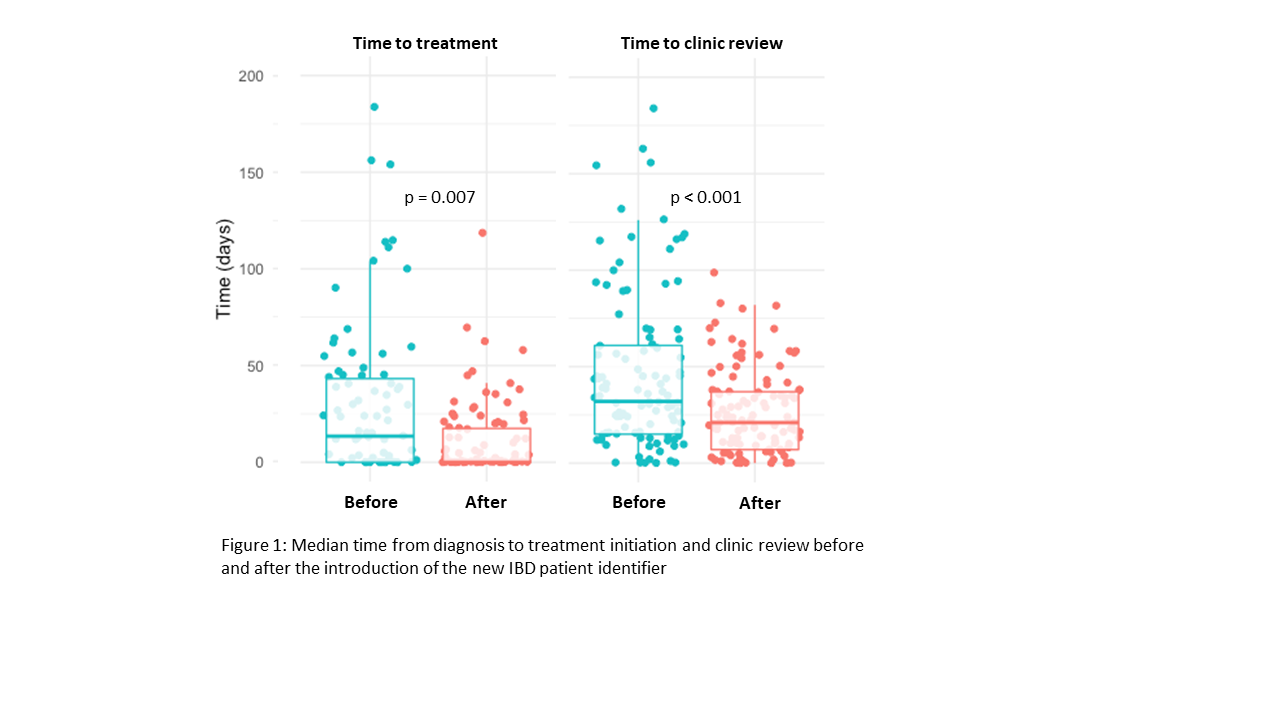
Conclusion
Systematic electronic screening of endoscopic reports linked to MDT review reduces the time to first treatment and specialist review in newly diagnosed patients with IBD.



