P257 The clinical decision support tool has low performance in predicting outcome to ustekinumab in Crohn’s disease
Alsoud, D.(1);Sabino, J.(1,2);Ferrante, M.(1,2);Verstockt , B.(1,2);Vermeire, S.(1,2);
(1)KU Leuven, Translational Research in Gastrointestinal Disorders- Department of Chronic Disease- Metabolism and Ageing, Leuven, Belgium;(2)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;
Background
Several biologicals and small molecules have been added to the therapeutic arsenal of inflammatory bowel disease, which generated a wide interest in precision medicine. In this context, both clinical features and laboratory tests have shown some correlation with therapy outcome. Recently, an online clinical decision support tool1 (CDST) was built, using clinical and biochemical variables (e.g. disease location, prior anti-TNF exposure), to help physicians predict the outcome of various biological treatments in Crohn’s disease (CD). We sought to evaluate the predictive performance of this tool in patients with CD initiating ustekinumab (UST).
Methods
Baseline data were collected from consecutive CD patients who started UST IV induction followed by eight weekly UST 90 mg SC maintenance from December 2015 through March 2021 at our referral center. The probability of achieving clinical remission (low, intermediate, or high) was determined using the online CDST using patients’ data. Also, differences in rates of primary non-response and ustekinumab persistence were compared between CDST classifications. Primary non-response was defined as failing to attain endoscopic response (≥50% decrease in SES-CD) nor clinical response (a drop in the physician global assessment score or patients reported outcome), both assessed at month 6 after UST initiation. Finally, we studied the relation between the individual components used to construct the CDST and the three classifications in a univariate analysis.
Results
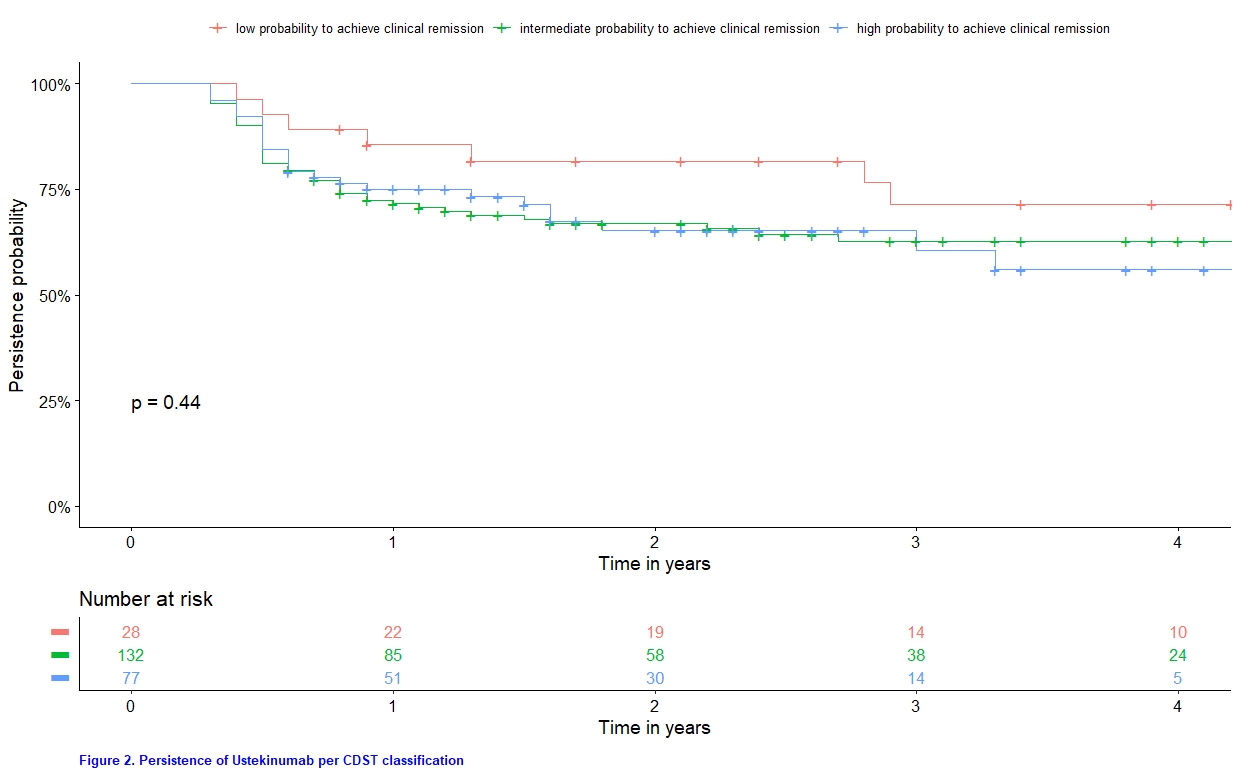
A total of 291 CD patients started UST, of whom 237 had objectively-assessed (CRP, fCal, symptoms or endoscopy) baseline disease activity. Using the CDST, the proportion of patients with “low”, “intermediate” or “high” probability to attain clinical remission were 11.8 % (n=28), 55.7 % (n=132) and 32.5 % (n=77), respectively. The CDST could not discriminate patients with primary non-response (p value = 0.18; Figure 1), nor identify trends in drug persistence (p value = 0.44; Figure 2). In the univariate analysis, 3 out of 9 components (active fistulizing disease, disease location and concomitant immunomodulatory use) were not significantly contributing to the CDST classification (Table 1).

Conclusion
The recently developed clinical decision support tool showed low performance in predicting short-term (primary clinical and endoscopic response) and long-term (drug persistence) real-life outcomes in CD patients starting UST. These findings underscore the necessity of incorporating molecular variables, in addition to clinical ones, to construct reliable and reproducible tools to predict therapeutic outcomes.
1. https://via.juxlyapps.com/pathway/archemedx/ibd-cdst/index.html#/disease-selection


