P261 Predictors and outcomes of ustekinumab dose escalation to every 4 or every 6 weeks in ulcerative colitis: a multicenter cohort study in the United States
Dalal, R.(1);Esckilsen, S.(2);Barnes, E.(3);Pruce, J.(1);Marcus, J.(1);Allegretti, J.(1);
(1)Brigham and Women's Hospital- Harvard Medical School, Gastroenterology- Hepatology and Endoscopy, Boston- MA, United States;(2)University of North Carolina at Chapel Hill, Medicine, Chapel Hill- NC, United States;(3)University of North Carolina at Chapel Hill, Gastroenterology and Hepatology, Chapel Hill- NC, United States
Background
Patients with ulcerative colitis (UC) on ustekinumab (UST) therapy may have suboptimal response to standard every 8 week (q8w) dosing. Empiric dose escalation to q4w or q6w is common, but the efficacy of these strategies are unknown in UC. We performed a multicenter cohort study to identify predictors and outcomes of UST dose escalation in UC.
Methods
This retrospective cohort study included adults initiating UST for UC (ICD-10-CM 51x) at three academic medical centers in the United States 1/1/2016-11/1/2021. Patients with prior colectomy were excluded. Disease activity was assessed using the 9-point Mayo score or simple clinical colitis activity index (SCCAI) in electronic health records. Independent variables included demographics, UC duration, extraintestinal manifestation, medication/substance use history, endoscopic extent/severity, reason for escalation, intravenous (IV) reinduction, dose interval, albumin, C-reactive protein, and bowel frequency. The primary outcome was steroid-free clinical remission (SCCAI/Mayo <3 points) 12-16 weeks after escalation. Secondary outcomes were clinical response (reduction in SCCAI/Mayo by ≥3 points from baseline) at 12-16 weeks and time to escalation. Additional endpoints included endoscopic response, failure within 16 weeks (treatment discontinuation or colectomy), improvement in fecal calprotectin, UC hospitalisation, and adverse events. Multivariable logistic and cox regression were used to identify factors associated with remission and time to escalation, respectively.
Results
108 UC patients initiated UST: 91.7% had prior anti-TNF exposure and 57.4% were taking oral steroids (Table 1). 39.6% (40/101 with data) achieved remission 12-16 weeks after induction. 42.6% (46/108) were escalated to q4w (n=33) or q6w (n=13) after median of 95 days (IQR 65-208 days) primarily for no/minimal response to induction (22/46) or loss of response (20/46) (Fig 1A). 55.0% (22/40 with data) achieved remission at 12-16 weeks, 67.5% (27/40) had response, 56.3% (9/16 with data) had endoscopic response (Table 2), and 30.0% (12/40) had treatment failure (Fig 1B). 10.0% (4/40) were hospitalised after median of 80 days and 5.0% (2/40) had adverse events (urinary tract infection and C. difficile infection). After multivariable analysis, lack of response to induction was inversely associated with remission after escalation. Bowel frequency and >2 prior biologics were associated with time to escalation (Table 3).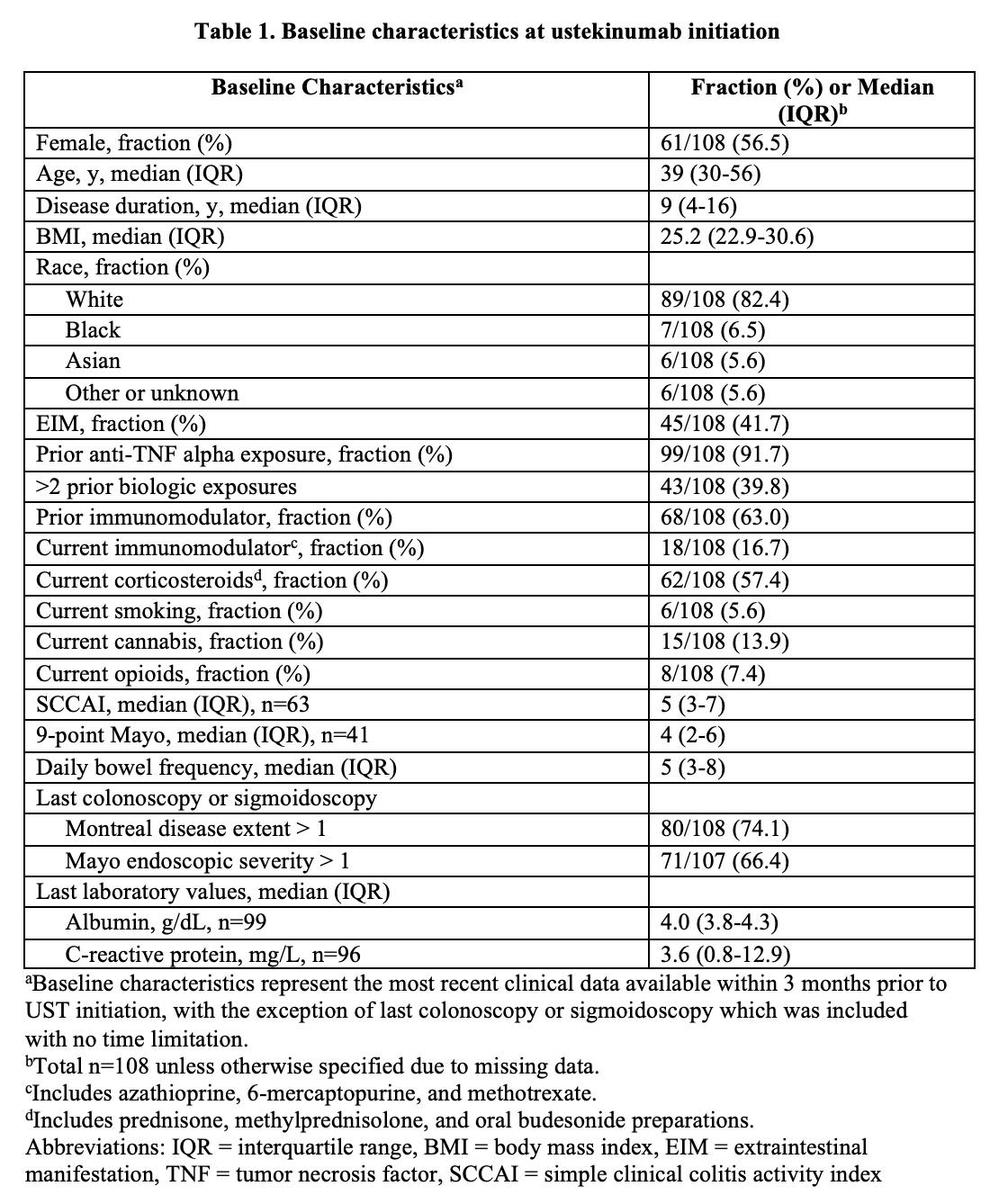



Conclusion
UST dose escalation resulted in clinical remission in >50% of UC patients and was more effective in those with loss of response compared to no/minimal response after induction. Prospective studies are needed to identify UC subpopulations that will benefit from various dosing strategies of UST.


