P272 Immune checkpoint inhibitor-related colitis assessment and prognosis: can inflammatory bowel disease scoring point the way?
V.T.F. Cheung1, T. Gupta1, A. Olsson-Brown2, S. Subramanian3, S.C. Sasson1, J. Heseltine2, E. Fryer4, E. Collantes4, J.J. Sacco2, M. Pirmohamed5, A. Simmons1, P. Klenerman1, B.P. Fairfax6, M.J. Payne6, M.R. Middleton6, O. Brain1
1Nuffield Department of Medicine, University of Oxford, Translational Gastroenterology Unit, Oxford, UK, 2Clatterbridge Cancer Centre, Oncology, Liverpool, UK, 3Royal Liverpool University Hospital, Gastroenterology, Liverpool, UK, 4John Radcliffe Hospital, Cellular Pathology, Oxford, UK, 5Institute of Translational Medicine, University of Liverpool, Molecular and Clinical Pharmacology, Liverpool, UK, 6Oxford Cancer Centre, Medical Oncology, Oxford, UK
Background
Immune checkpoint inhibitors (ICI) have revolutionised oncological care but cause immune-related adverse events (irAE). Inflammation of the gastrointestinal tract (enterocolitis) is the most frequent cause of significant morbidity and cessation of ICI. We sought to determine whether Common Terminology Criteria for Adverse Events (CTCAE) classification of colitis, inflammatory bowel disease (IBD) biomarkers, endoscopic scores and histology scores correlate with disease outcome.
Methods
A retrospective study was performed on patients receiving ICI for metastatic melanoma, non-small cell lung cancer and urothelial cancer from 2012 to 2018 at two UK centres by reviewing the electronic patient records and oncology prescribing databases to identify patients who had irAE colitis. irAE colitis was defined as diarrhoea requiring steroid/infliximab therapy for resolution and/or with endoscopic/histological confirmation. Demographics, clinical data, endoscopic findings, histopathology reports and treatment outcomes were obtained. Patients were divided into three categories: (1) Mild-moderate disease where diarrhoea settled rapidly following a course of steroids ≤60 days; (2) Refractory or moderate–severe colitis requiring steroids >60 days; and (3) Severe colitis requiring infliximab ‘rescue therapy’. Endoscopies were re-analysed with image scoring using Mayo and ulcerative colitis Endoscopic Index of Severity (UCEIS) scores by two independent endoscopists. Histology was scored using the Nancy Index by two expert GI histopathologists.
Results
1074 patients were analysed. Twelve per cent (134) developed irAE colitis. Median patient age was 66, 59% were male. Age, sex, smoking status and prior IBD or autoimmune disease were not associated with risk of developing irAE colitis. CTCAE grading did not correlate with steroid duration (
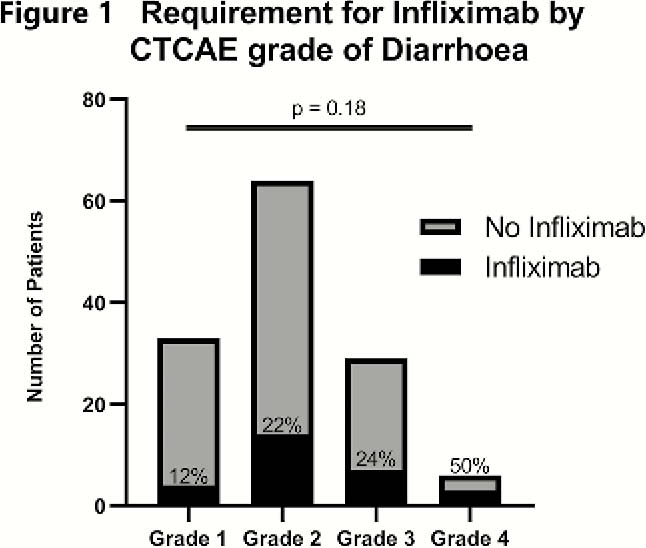
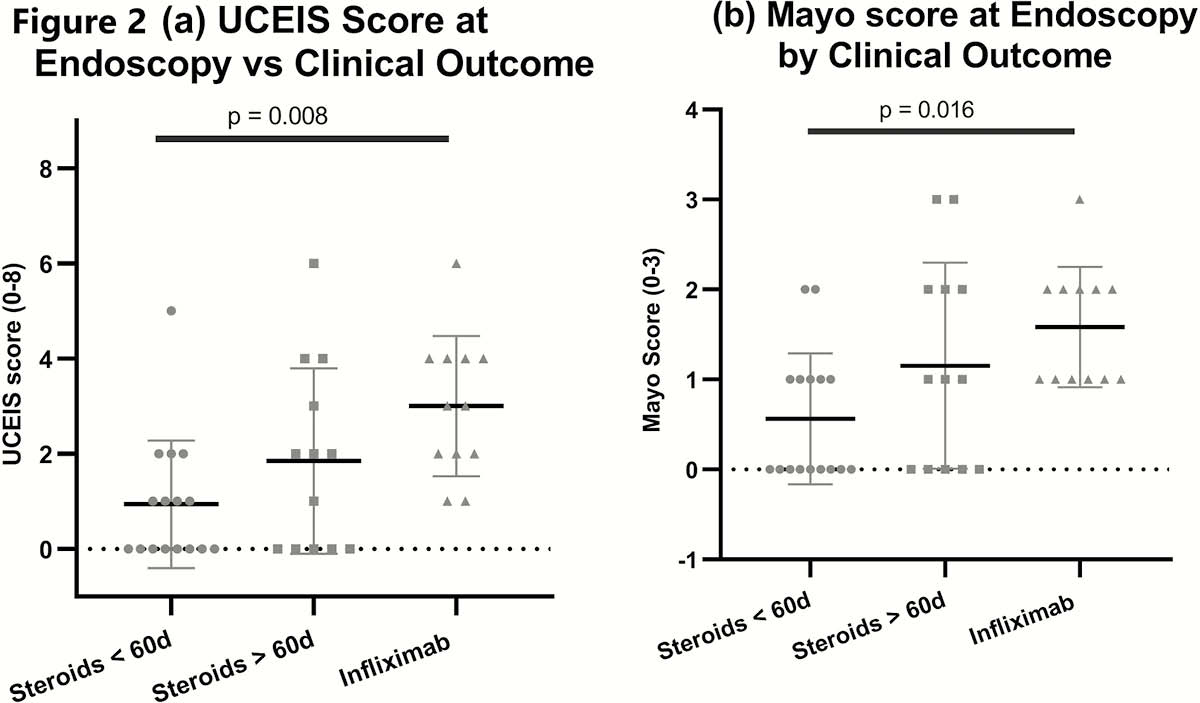
22% of patients received rescue therapy with infliximab either as single or multiple doses, with 3 undergoing a colectomy (2.3%; two had ipilimumab and one had combination). The UCEIS (
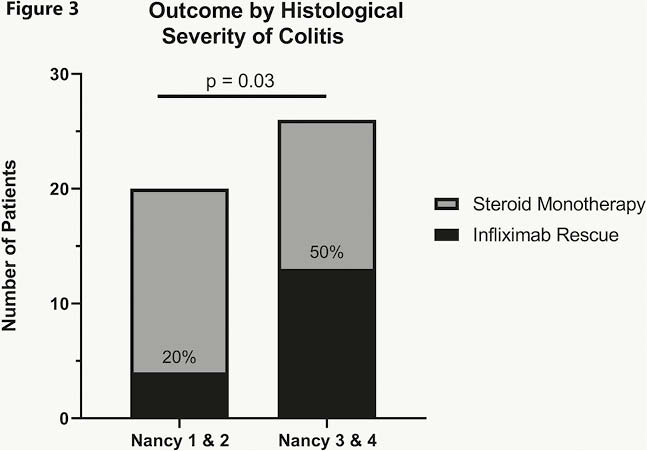
Conclusion
Clinical assessment with CTCAE does not accurately reflect immunotherapy-colitis severity thus negatively impacting timely treatment decisions. Our data support endoscopic scoring of colitis severity, and demonstrate the potential prognostic utility of objective histologic scoring. we suggest that clinical guidelines are changed to incorporate new algorithms for the investigation and management of irAE colitis.


