P280 Introduction of subcutaneous infliximab CT-P13 and vedolizumab in daily clinical practice: outstanding questions demonstrate the need for post-marketing studies
Ferrante, M.(1,2);Sabino, J.(1,2);Lobaton, T.(3);Dreesen, E.(4);Hoefkens, E.(5);Fierens, L.(2);Liefferinckx, C.(6);
(1)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(2)Catholic University of Leuven, Department of Chronic Diseases- Metabolism and Ageing, Leuven, Belgium;(3)University Hospital Ghent, Department of Gastroenterology, Ghent, Belgium;(4)Catholic University of Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium;(5)Imelda General Hospital, Department of Gastroenterology, Bonheiden, Belgium;(6)Erasme Hospital ULB, Department of Gastroenterology, Brussels, Belgium
Background
Although clinical trials led to the registration of subcutaneous (SC) infliximab CT-P13 (IFX) and SC vedolizumab (VDZ) for patients with Crohn’s disease (CD) and ulcerative colitis (UC), many practical aspects have not been addressed yet. We explored what strategy Belgian clinicians plan to follow once these formulations become available.
Methods
A 3-round Delphi process was initiated in Jan 2021. A core panel (4 IBD clinicians, 1 PKPD expert, 1 IBD nurse, 1 methodologist) developed a neutral video on the available data, as well as 65 statements and 9 multiple-choice questions related to the practical use of SC IFX and VDZ. The expert panel, consisting of 45 IBD clinicians (median [IQR] years of practice 19 [11-26] years), scored all statements on a 10-point Likert scale. We here present the results of the first Delphi round. The complete report will be available after the final Delphi round in May 2021. A ≥70% consensus level (scores 1-4 for disagreement, scores 7-10 for agreement) is foreseen.
Results
The majority of IBD clinicians expressed the need for more scientific data on switching from intravenous (IV) to SC therapy (Table 1). Furthermore, a minority would consider starting SC therapy after only two IV induction doses (Figure 1).
Most clinicians would restrict switching to patients who achieved both clinical, biological response, and endoscopic response (Figure 2) and this only when receiving standard IV dosing of 5mg/kg IFX (75.5%) or 300mg VDZ every 8 weeks (68.9%). Both for IFX and VDZ, 57.1% of IBD clinicians would administer a first SC dose no later than 6 weeks after the last IV administration.
The IBD clinicians highlighted the role of the IBD nurse in explaining and guiding a potential switch (82.2% IFX, 88.9% VDZ) and supervising the first SC administration (100.0% IFX, 95.5% VDZ). They emphasized the need for a clinical follow-up within 8 weeks (88.6% IFX, 88.9% VDZ).
Although reactive therapeutic drug monitoring under SC therapy was considered helpful by IBD clinicians (77.8% IFX, 68.9% VDZ), they acknowledged the lack of an optimal serum concentration (73.4% IFX, 84.3% VDZ).
Round one did not result in a consensus on using SC formulations in patients with quiescent perianal fistulising disease or a prior episode of acute severe UC. Finally, the optimal strategy in case of an objectified clinical relapse after switching to SC therapy seems is clearly unknown (83.3% IFX, 76.2% VDZ).
Conclusion
Although both SC IFX and SC VDZ will become available soon, the majority of IBD clinicians expressed the need for more scientific data on switching from IV to SC therapy. This Delphi process may indicate pivotal remaining research questions to be answered through post-marketing studies.
Table 1 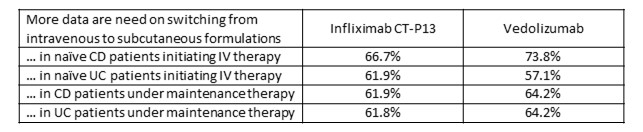
Figure 1 
Figure 2


