P283 Infliximab re-challenge: could it be an option in refractory IBD patients?
Truyens, M.(1);Pijoan Comas, E.(2);Glorieus, E.(1);Geldof, J.(1);Lobatón Ortega, T.(1);
(1)University Hospital of Ghent, Department of Gastroenterology and Hepatology, Ghent, Belgium;(2)Palamós Hospital, Department of Gastroenterology and Hepatology, Palamós, Spain;
Background
Infliximab (IFX) is effective in treatment of inflammatory bowel disease (IBD). However, primary non-response and secondary loss-of-response rates go up to 30-40% and intolerance may occur leading to treatment switch. Unfortunately, even with increased availability of new molecules, some patients remain refractory to all currently available medical options. This study assesses the effect of IFX re-challenge in a real-life cohort of highly refractory IBD patients.
Methods
A retrospective observational study was set up to analyse the effect of IFX rechallenge in IBD patients previously treated with IFX and found refractory to other biologics. Cases between August 2018 and June 2021 with at least one year of follow-up (FU) were analysed. Clinical, biological (CRP and faecal calprotectin [FC]) and endoscopic response was evaluated, as well as adverse events (AE).
Results
Ten patients with refractory IBD were included (9 Crohn’s disease [CD], 1 ulcerative colitis [UC]). Details on previous treatment before IFX re-challenge are shown in Table 1.
All patients had active disease at the start of IFX re-challenge. Median baseline CRP was 12.6mg/L [4.9-24.1] and FC 396mg/kg [64-2786.8]. IFX was started in combination with systemic steroids, immunosuppressants and ustekinumab in 50%, 70% and 10% of patients, respectively. Baseline endoscopy showed severe active disease in 7/8 patients.
Clinical and endoscopic response rates are shown in Table 2. Biological response is shown in Figure 1. After 1 year FU, all (7/7) required intensified dosing (4-weekly), in 3/7 corticosteroid free clinical remission was reached. IFX re-challenge was discontinued after the second administration in 2/10, due to a severe allergic reaction despite premedication with antihistamines and corticosteroids (1 anaphylactic shock admitted to intensive care unit, fully recovered). IFX was continued in 8/10.
Other AE (not in all cases considered to be related to IFX) included: disease flare-up requiring hospitalization (3/10), liver test abnormalities (1/10), new perianal abcedation (1/10), subobstruction (1/10), C. difficile infection (1/10), EBV infection (1/10) and Addison crisis (1/10).

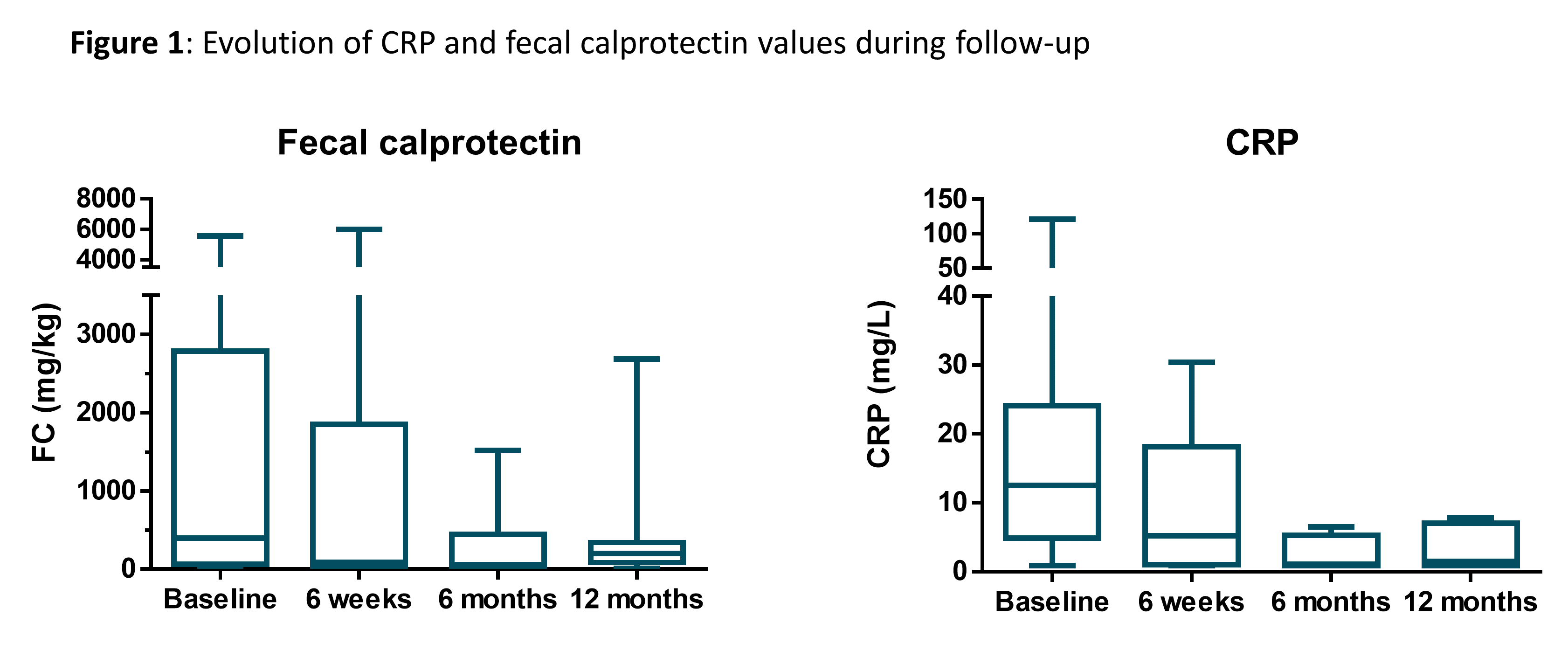
Conclusion
IFX re-challenge was efficient in inducing clinical and endoscopic response in this highly refractory IBD population. However, there is a non-negligible risk of AE including infusion reaction.


