P298 Pregnancy in Crohn's and Colitis - Observations, Levels and Outcomes Extension (PICCOLO-X) Study: Biological therapy reduces faecal calprotectin and the need for corticosteroids in pregnant women with Inflammatory Bowel Disease
Prentice, R.(1);Wright, E.(2);Flanagan, E.(2);Goldberg, R.(1);Prideaux, I.(1);Ross, A.(2);Burns, M.(1);Bell, S.(1);
(1)Monash Medical Centre, Gastroenterology, Melbourne, Australia;(2)St Vincent's Hospital Melbourne, Gastroenterology, Melbourne, Australia; Pregnancy in Crohn's and Colitis: Observations Levels and Outcomes Study Group
Background
Active Inflammatory Bowel Disease (IBD) in pregnancy is associated with adverse obstetric and neonatal outcomes. PICCOLO-X is an ongoing prospective cohort study of pregnant women with IBD, aiming to describe rates of biochemically active IBD in the current therapeutic landscape, and associations with adverse outcomes.
Methods
Participants were evaluated preconception, in each trimester and postpartum with clinical and biochemical data collected. Univariate and multivariate logistic regression was performed to identify the relative risk (RR) of adverse outcomes by relevant exposures. Fischer's exact test was used to compare group medians.
Results
164 completed pregnancies were included, with 62% exposed to biologic therapy and 56% having Crohn’s Disease (Table 1). 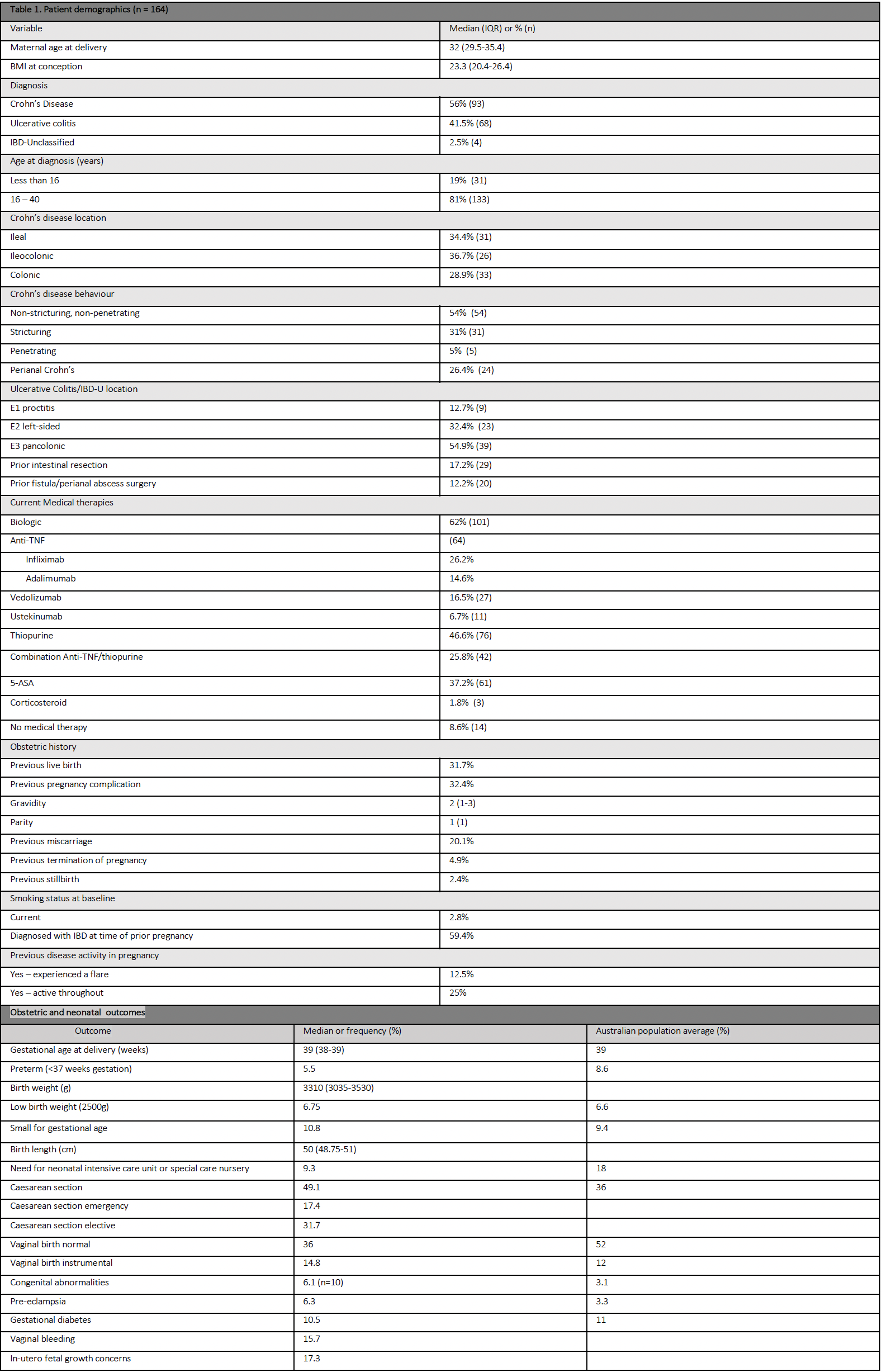
Clinically active disease (PGA ≥1) was reported in 23.9% in trimester 1 (T1), 18.8% in trimester 2 (T2) and 17.5% in trimester 3 (T3), and was less common in biologic exposed patients (Table 2 & 3). 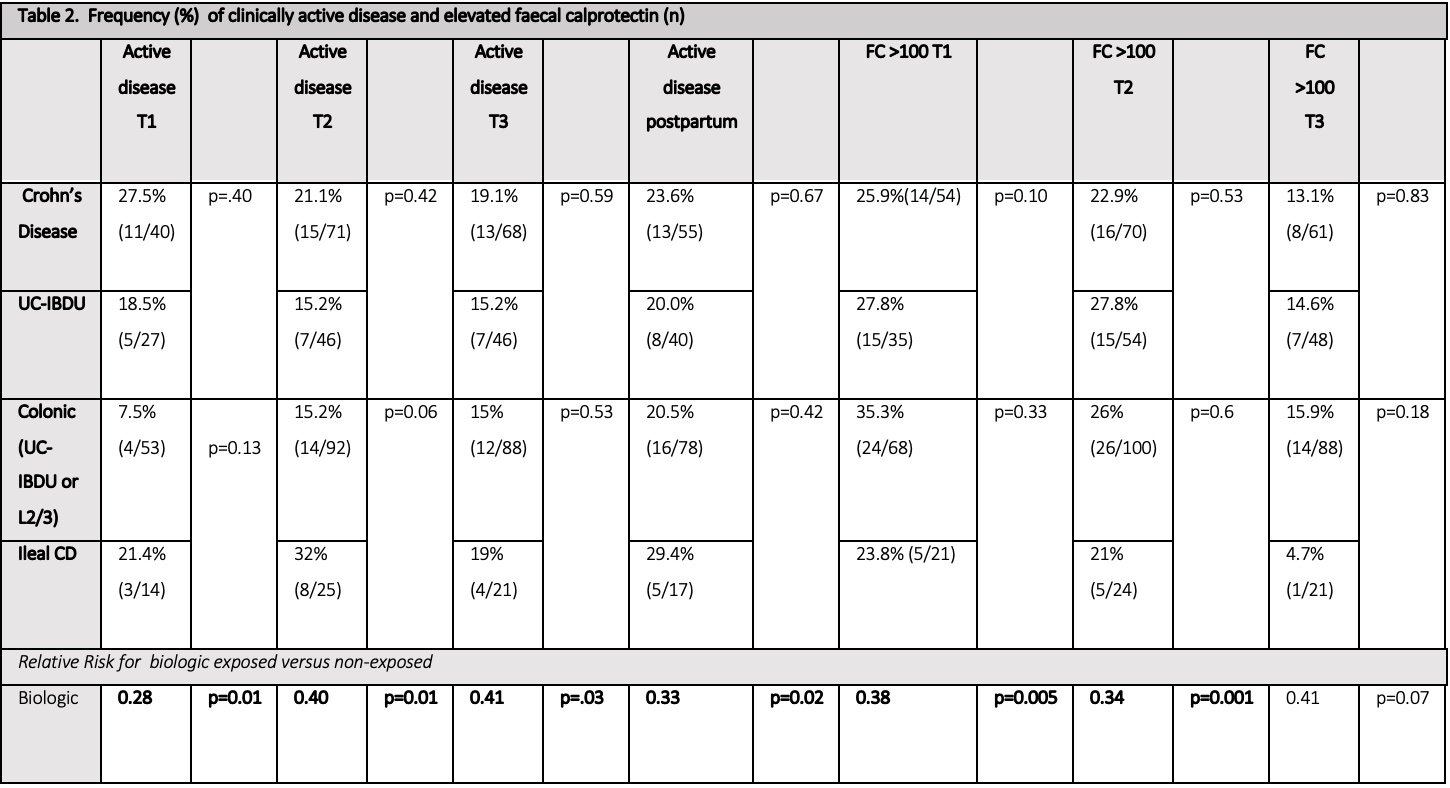

The median faecal calprotectin (FC) was <50μg/g in all trimesters, and was lower in those receiving biologics (24.9 vs 86μg/g T1 p=0.02, 26.25 vs 46μg/g T2 p=0.02, 30 vs 58μg/g T3 p=0.04) (Table 3). Adverse pregnancy outcome rates were equivalent to the normal population (Table 2). Corticosteroid (CCS) exposure in T2 was associated with an increased risk of preterm delivery (PTD) on multivariate analysis (RR 8.12, 95%CI 1.23-53.72, p=0.03). T1 FC ≥250μg/g (RR 2.41 95% CI 0.5-4.31, p=0.01), clinically active disease in T3 (RR 4.7, 95% CI 1.02-26.61, p<0.01) & T3 FC 100-250 (RR 6.8 95% CI 1.71-26.0, p<0.01), but not T3 FC ≥250μg/g (RR 1.6, 95% CI 0.41-8.25, p=0.6) were associated with an increased risk of PTD on univariate analysis (Table 4). 
Biologic exposure was associated with a reduced need for CCS; no participants on a biologic had CCS in T1, with RR 0.14 (95% CI 0.03-0.66, p=0.01) in T2 and 0.11 (95% CI 0.02-0.46, p=0.003) in T3. On multivariate analysis, FC >100μg/g in T2 was associated with an increased risk of emergency caesarean section (RR 1.65, 95% CI 0.47-2.82, p<0.01), whilst FC >100μg/g in T2 (RR 1.65 95% CI 0.4-2.91 p=0.01) and T3 (RR 2.47,95% CI 1.36-8.82, p<0.01) were associated with an increased risk of neonatal intensive care unit admission.
Conclusion
PICCOLO X describes low rates of biochemical and clinical active disease in pregnant women with IBD as compared to historical cohorts, possibly due to the cohort’s high rate of biologic use. Pregnancy outcomes were favourable overall. Clinical and biochemical inflammation and CCS exposure were associated with an elevated risk of adverse pregnancy outcomes. Biologic therapy reduces FC and the need for steroids.


