P300 Crohn's Disease Strictures Respond to Drug Treatment and Treat-to-Target Intense Combination Therapy is More Effective than Standard Anti-TNF Therapy. The STRIDENT Randomised Controlled Trial
Schulberg, J.(1,2);Wright, E.(1,2);Holt, B.(1,2);Sutherland, T.(2,3);Ross, A.(1);Hamilton, A.(1,2);Vogrin, S.(2);Miller, A.(1);Connell, W.(1);Lust, M.(1);Ding, N.(1,2);Moore, G.(4);Bell, S.(4);Shelton, E.(4);Christensen, B.(5);De Cruz, P.(6);Rong, Y.(7);Kamm, M.(1,2);
(1)St. Vincent's Hospital Melbourne, Department of Gastroenterology, Melbourne, Australia;(2)University of Melbourne, Department of Medicine, Melbourne, Australia;(3)St. Vincent's Hospital, Department of Radiology, Melbourne, Australia;(4)Monash Health and Monash University, Department of Gastroenterology, Melbourne, Australia;(5)The Royal Melbourne Hospital, Department of Gastroenterology, Melbourne, Australia;(6)Austin Health, Department of Gastroenterology, Melbourne, Australia;(7)Latrobe Regional Hospital, Department of Gastroenterology, Traralgon, Australia STRIDENT
Background
Strictures are the commonest complication in Crohn’s disease. Surgery and endoscopic dilation are the main treatments; drug therapy has been considered contra-indicated. Given that most strictures have an inflammatory component we aimed to assess the efficacy of anti-inflammatory therapy, and to identify the optimal treatment.
Methods
In this randomised trial patients with symptomatic Crohn’s disease strictures and inflammation were assessed by imaging (MRI, colonoscopy, intestinal ultrasound) and for inflammation (faecal calprotectin and CRP). Symptoms were assessed using an Obstructive Symptom Score (OSS). Patients with short endoscopically-accessible strictures had a baseline endoscopic balloon dilation if indicated. Patients were then randomised 2:1 to high dose adalimumab induction (160mg weekly for 4 weeks) with 40mg fortnightly maintenance plus thiopurine, with therapy increased for ongoing inflammation at 4 and 8 months, versus standard dose adalimumab mono-therapy. At 12 months primary endpoint was improved OSS. Secondary outcomes: disease activity, treatment failure, stricture morphology, inflammation, psychological well-being, disability, and quality of life. MRI was assessed blindly.
Results
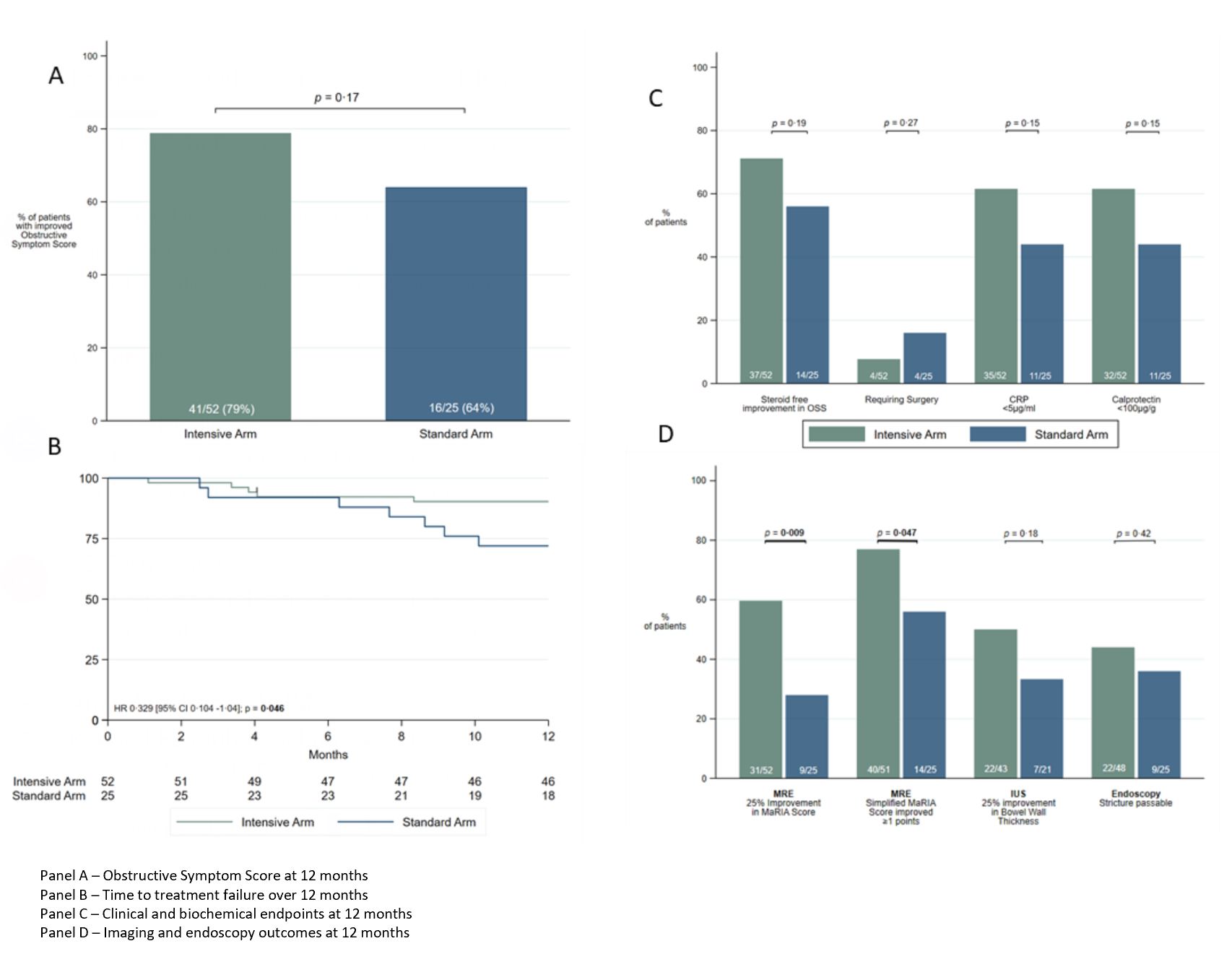
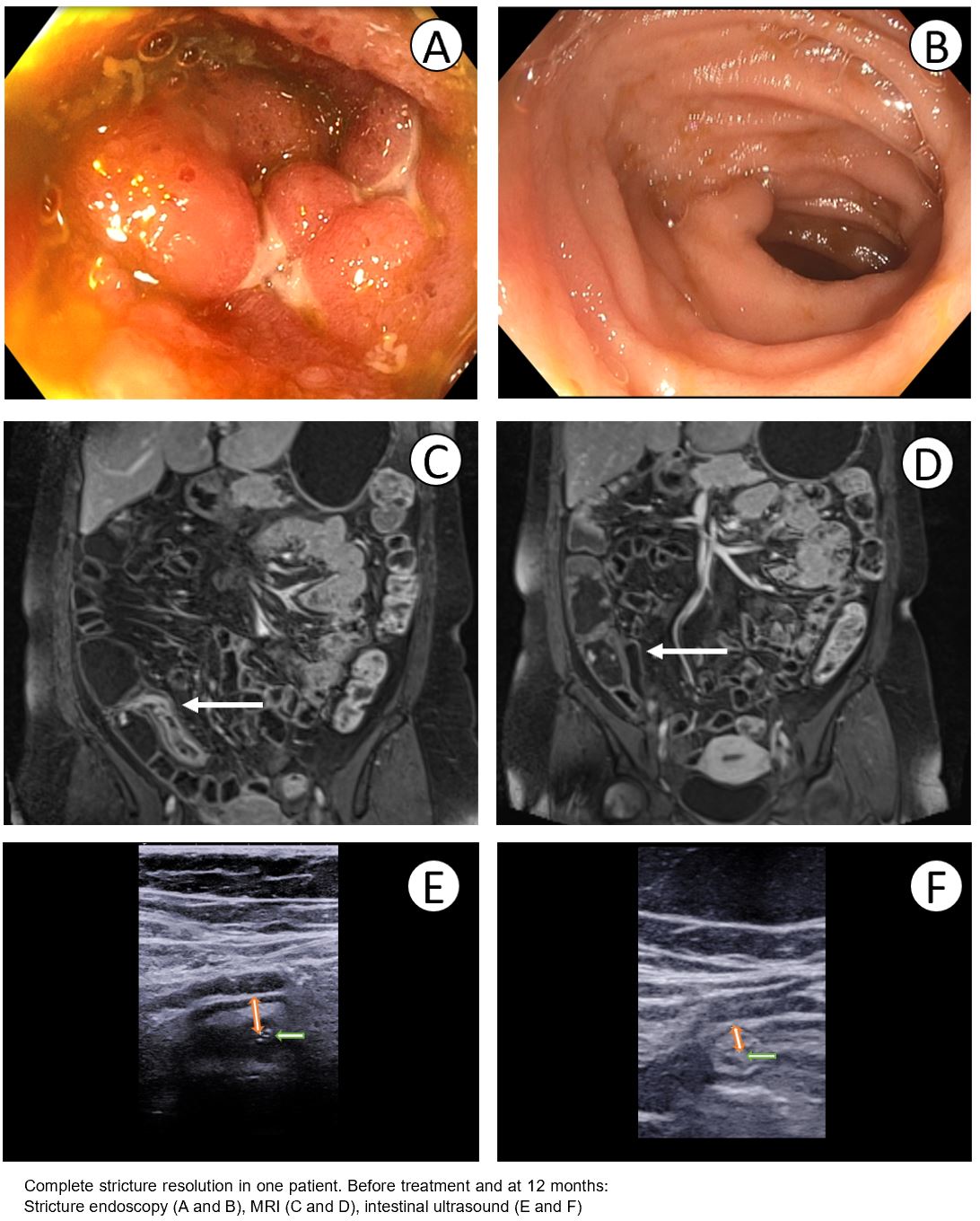
52 patients were randomised to the intensive and 25 to the standard treatment arm. 27 of 52 (52%) intensive treatment patients dose escalated at 4 or 8 months. Improved OSS at 12 months occurred in 41 (79%) intensive treatment and 16 (64%) standard treatment arms (P=0.17). Treatment failure was less common in the intensive treatment arm (10%) versus the standard treatment arm (28%) (P=0·045). Faecal calprotectin normalised (<100mcg/g) in 32 (62%) v 11 (44%) (P=0.15), and CRP normalised in 32 (62%) v 11 (44%) (P=0.15), in intensive versus standard treatment arms respectively. MRI stricture morphology improvement (MaRIA score decrease ≥25%) was seen in 31 (61%) v 9 (28%) (P=0.009) and in 40 (78%) v 14 (56%) using the simplified MaRIA score (≥1 point improvement) (P=0.047). Improvement in bowel wall thickness by >25% on ultrasound was seen in 22/43 (51%) and 7/21 (33%) respectively (P=0.18). MRI complete stricture resolution was seen in 10/51 (20%) and 4/25 (16%) (P=0.7). At 12 month colonoscopy 22/48 (46%) v 9/25 (36%) strictures were passable (P=0.42). 12 month median drug levels were 13.2µg/ml and 6.6µg/ml respectively (P<0.0001). See summary results figure 1 and case example figure 2.
Conclusion
Crohn’s disease strictures are responsive to drug therapy. A majority of patients experience symptom improvement and many have improved stricture morphology. Treat-to-target therapy intensification results in less treatment failure, less stricture-associated inflammation, and greater improvement in stricture morphology.