P302 Diagnostic accuracy of a serum-based biomarker panel for endoscopic activity in ulcerative colitis
A. HOLMER, B. Boland, S. Singh, H. Le, J. Neill, A. Miralles, A. Collins, W. Sandborn, P. Dulai
Department of Gastroenterology, University of California San Diego, San Diego, USA
Background
The endoscopic healing index (EHI, Monitr, Prometheus Biosciences, San Diego, CA) is a serum-based biomarker panel available for identifying mucosal inflammation in Crohn’s disease.[1] We aimed to study its performance for identifying mucosal inflammation in ulcerative colitis.
Methods
EHI was analysed on serum samples paired with endoscopies from adult patients (≥18 years) participating in a prospective biobank (June 2014 to December 2017). Area under receiver operating characteristic curves (AUROC) were used to assess the accuracy of EHI for endoscopic improvement (EI; Mayo endoscopic sub-score [MES] 0–1) and endoscopic remission (ER; MES 0). Sensitivity for EHI was calculated using a cut-off previously identified for Crohn’s disease which optimised performance for ruling out endoscopic activity (20 points). Alternative cut-offs were explored.
Results
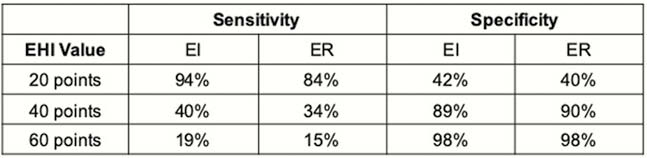
A total of 114 patients were included, with an overall prevalence of 56% and 44% for EI and ER. The AUROC was 0.79 (95% CI 0.70–0.87) for EI and 0.70 (95% CI 0.61–0.80) for ER. A cut-off of 20 points had a sensitivity of 94% (95% CI 83–99%) for ruling out moderate to severe (MES 2–3) endoscopic activity, and a sensitivity of 84% (95% CI 72–92%) for ruling out mild to severe (MES 1–3) endoscopic activity. A cut off of 40 points or higher had > 90% specificity for ruling in moderate to severe (MES 2–3) or mild to severe (MES 1–3) endoscopic activity. (Table 1)
 |
Conclusion
EHI has favourable accuracy in identifying the presence of mucosal inflammation in patients with ulcerative colitis. Although it was not developed and validated for ulcerative colitis, further validation is warranted.
D’Haens G et al. Development and validation of a test to monitor endoscopic activity in patients with crohn’s disease based on serum levels of proteins.


