P304 Rational infliximab induction dosing to achieve long-term deep remission in children with Inflammatory Bowel Diseases
Kantasiripitak, W.(1);van Hoeve, K.(2);Sabino, J.(3,4);Vermeire, S.(3,4);Hoffman, I.(2);Declerck, P.(1);Thomas, D.(1);Ferrante, M.(3,4);Dreesen, E.(1,5);
(1)KU Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium;(2)University Hospitals Leuven, Department of Paediatric Gastroenterology Hepatology and Nutrition, Leuven, Belgium;(3)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(4)KU Leuven, Department of Chronic Diseases Metabolism and Ageing, Leuven, Belgium;(5)Uppsala University, Department of Pharmacy, Uppsala, Sweden
Background
Adequate infliximab (IFX) trough concentrations (TCs) during induction treatment are predictive for long-term clinical and endoscopic remission in paediatric patients with inflammatory bowel diseases (IBD). However, under the approved weight-based dosing (5 mg/kg), children often have low IFX TCs, since the relationship between bodyweight and the IFX pharmacokinetic (PK) parameters is nonlinear. Therefore, there is a need to optimise paediatric dosing to engage optimal IFX TCs during induction treatment.
Methods
Fifty-two paediatric patients with IBD (34 Crohn’s disease (CD), 18 ulcerative colitis (UC)) contributed IFX samples (246 intermediate and 150 trough samples) during either induction treatment (n=32) or maintenance treatment (n=20). A population PK (popPK) model was developed to describe the relationship between IFX dose and exposure. The previously published IFX popPK model based on data of 112 children with CD in the Phase 3 REACH trial was used as a frequentist prior to inform parameter estimation using NONMEM. Our popPK model was used to simulate IFX TCs during induction treatment under different induction dosing regimens (5 mg/kg, 7.5 mg/kg, and 10 mg/kg IFX at weeks 0, 2, and 6). Probabilities of TC target attainment (PTA) were compared. The TC target, associated with a 75% probability of achieving deep remission (DR) at six months after initiating IFX treatment, was identified using a logistic regression model and a ROC analysis. DR was defined as combined endoscopic remission (Simple Endoscopic Score for CD <3 or Mayo endoscopic sub-score 0) and corticosteroid-free clinical remission (Paediatric CD or UC Activity Index <10).
Results
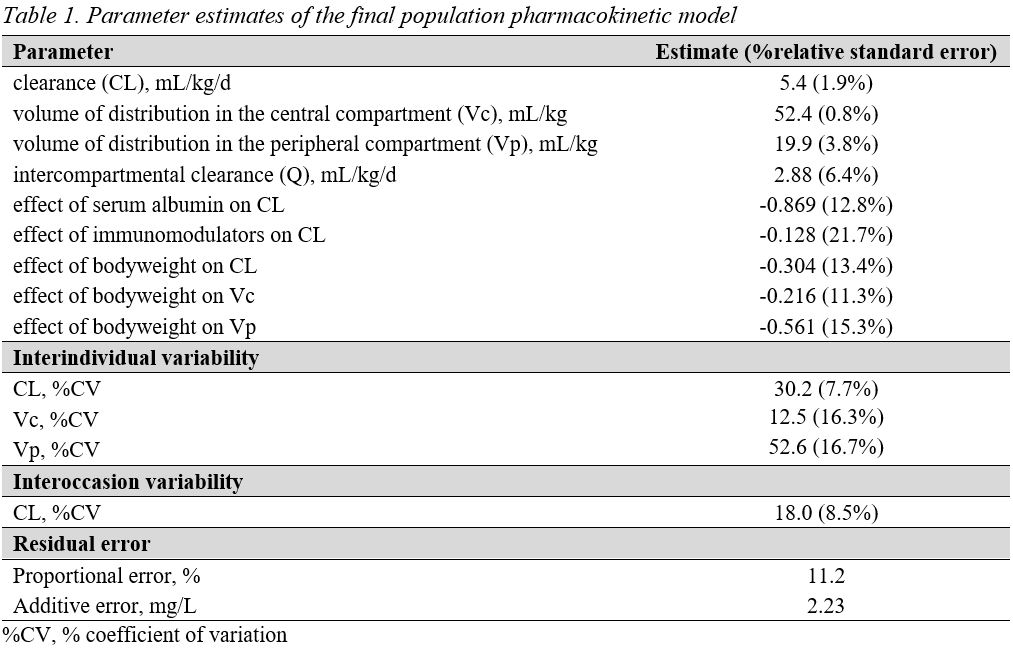
The median boyweight was 44 kg (range 15-92 kg). A 2-compartment popPK model with first-order elimination described IFX concentrations well (Table 1). The IFX clearance by weight increased with decreasing bodyweight, decreasing serum albumin and absence of immunomodulator combo-therapy. Also, the IFX volume of distribution in the central and peripheral compartment by weight increased with decreasing bodyweight. A total of 18/32 (56%) patients in the induction treatment achieved DR at six months. An IFX TC of 23.0 mg/L at week 6 was identified as the TC target (100% sen, 33% spe, 100% npv, and 50% ppv). Children with a bodyweight less than 30 kg could only reach a 50% PTA when receiving 10 mg/kg IFX combo-therapy during induction (Figure 1). While children with a bodyweight above 30 kg had more than 50% PTA when receiving either 10 mg/kg IFX monotherapy or 7.5 mg/kg IFX combo-therapy (Table 2).
Conclusion
IFX doses higher than 5 mg/kg are needed during induction in children with IBD to facilitate the attainment of the TC target, thereby increasing the chance of long-term DR.