P315 Population pharmacokinetics and pharmacodynamics of ozanimod in ulcerative colitis
Tatosian, D.(1);Shen, J.(1);Chen, L.(1);Lavigne, J.(2);Teuscher, N.(3);Harris, S.(1);Chitkara, D.(1);Tirucherai, G.S.(1);Marta, C.(1);
(1)Bristol Myers Squibb, Research and Development, Princeton, United States;(2)Certara USA- Inc., Integrated Drug Development, Montréal, Canada;(3)Certara USA- Inc., Integrated Drug Development, Raleigh, United States
Background
Ozanimod is an oral small molecule sphingosine 1-phosphate receptor modulator approved for relapsing forms of multiple sclerosis (RMS) and under development for ulcerative colitis (UC) and Crohn’s disease. Ozanimod and its major active metabolites CC112273 and CC1084037 block lymphocyte egress from lymphoid tissues, reducing the absolute lymphocyte count (ALC) in blood. To characterise the influence of patient characteristics on ALC response, a population PK/PD analysis was conducted.
Methods
A nonlinear mixed-effects Emax model described the ALC time course and relationship to CC112273 concentrations in healthy subjects and patients with UC or RMS. The analysed data included 34,449 ALC values obtained from 4122 subjects enrolled in 11 Phase 1, 2, and 3 clinical studies. The effect of age, sex, smoking status, disease type, body weight, total bilirubin, and baseline ALC on the maximum ALC reduction (Emax) and half-maximal concentration (EC50) were investigated. Simulations of typical ALC reductions for healthy subjects and patients with UC or RMS receiving 0.5 or 1 mg ozanimod were conducted to explore the influence of smoking and assess the time for ALC recovery.
Results
Increasing CC112273 concentrations were associated with reductions in ALC to a maximum of 74% from baseline, with an estimated EC50 of 2.8 nM CC112273. Healthy subjects, females, and higher body weights were associated with larger ALC Emax (Figure 1), while healthy subjects, increased age, nonsmokers and higher baseline ALC were associated with an increased EC50 (Figure 2). UC patients receiving 0.5 mg and 1 mg ozanimod HCl are predicted to have mean ALC reductions of 46.1% and 57.2%, respectively, with similar reductions for RMS patients of 48.0% and 57.4%. Smoking was associated with a 27.2% reduction in the ALC EC50, which partially offsets the reduced plasma CC112273 concentrations in smokers. Simulations show that 1 mg ozanimod has comparable ALC reductions of 57.4% in nonsmokers and 52.7% in smokers. Upon discontinuing ozanimod 1 mg in UC patients, a mean recovery time to normal (1 x 109/L ALC count) of 32.8 days was predicted, with 90% of the population recovering in 3 months. Overall results were consistent with findings from clinical trials.
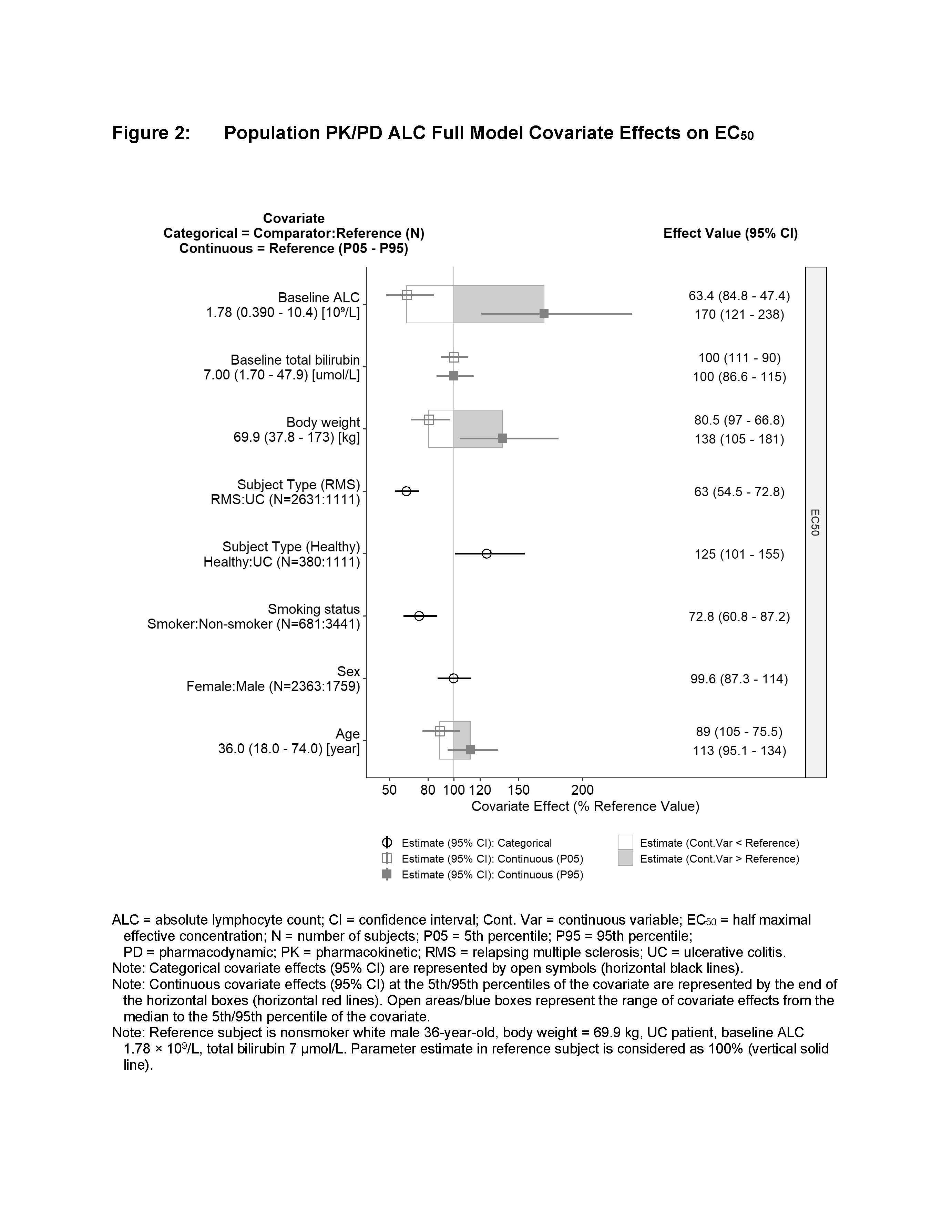
Conclusion
Population analysis demonstrated a similar PK/PD relationship for ALC reductions in patients with RMS and UC. Smoking was not associated with substantial difference in ALC, consistent with no loss of efficacy in smokers. Upon discontinuation of ozanimod treatment, the majority of UC and RMS patients will recover lymphocyte counts to normal within 3 months.


