P319 Incorporating HLADQA1*05 in pre-biologic screening in IBD patients initiating biologic therapies
Aleman Gonzalez, H.(1);Ramachandran, S.(1);Whitehead, E.(1);Pattinson, A.(1);Stamp, K.(1);Turnbull, J.(1);Myers, S.(1);Talbot, A.(1);Sebastian, S.(1);
(1)Hull University Teaching Hospitals, Inflammatory Bowel Disease, Kingston Upon Hull, United Kingdom;
Background
The PANTS study reported high risk of immunogenicity and loss of response in anti Tumor Necrosis Factor (anti-TNFs) treated Crohn’s disease (CD) patients carrying HLADQA1*05 allele. The proposed biomarker stratified trial to evaluate the usefulness of HLA testing prior to initiation of anti-TNFs is not yet available. We aimed to evaluate the use of HLADQA1*05 as part of pre-biologic screening in IBD patients initiating biologics on MDT decision on drug choice and disease outcomes
Methods
We prospectively included all IBD patients who had HLADQA1*05 tested prior to initiation of biologics over a period of 12 months. Patients with definitive indication for one class of drug or drug strategy (perianal fistula, acute severe colitis, contraindications to infliximab, co-existent EIMs) were excluded. Primary outcome was treatment persistence at 6 and 12 months. Secondary outcomes were steroid free remission at 6 and 12 months, use concomitant immunosuppression and proportion needing dose escalation.
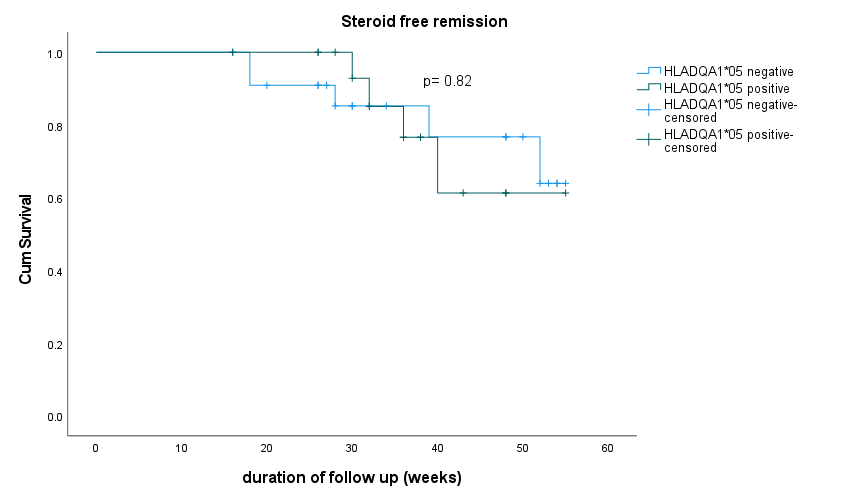
Results
Seventy-six patients were included in analysis (UC= 32, CD=43, IBD-U =1). HLADQA1*05 was positive in 46.7% of patients. The therapy class choice was as recorded in figure 1. Concomitant immunosuppression was used in 44% of the whole cohort and in 100% of HLADQA1*05 positive patients started on anti-TNF agents. Primary non-response was recorded in 8 patients and secondary loss of response in 3 patients. Among patients started on anti-TNFs, anti-drug antibodies were detected in 10 (15.6%) patients with 7 out of 10 positive for HLADQA1*05. However, only 3 (4.6%) had undetectable drug levels in the presence of antibody and all three were HLADQA1*05 positive. Two patients had reactions during induction therapy both were HLADQA1*05 positive and were on combination therapy with Infliximab. Therapy persistence with initial drug strategy and steroid free remission at 6 months was recorded in 77.1% and 78% respectively. There was no significant difference in drug persistence rates at 6 months and 12 months in patients with HLADQA1*05 variant or those with variant absent (Figure 2). Steroid free remission at 6 and 12 months was also similar irrespective of the variant status (Figure 3)

Conclusion
Choice of therapy incorporating HLADQA1*05 status may allow anti-TNF monotherapy and tailoring of therapy in IBD patients. A randomised stratified biomarker trial is required to determine the utility of pre-treatment testing.


