P321 Mucosal Healing and Clinical remission after Treatment with Adalimumab in Small Intestinal Crohn’s Disease (SIMCHA study): final results from a prospective, open-label, single-arm study
Wetwittayakhlang, P.(1);Golovics , P.(1);Hahn, G.(1);Bessissow, T.(1); Afif, W.(1);Wild, G.(1);Bitton, A.(1);LakatosPhD, P.L.(1);
(1)Mcgill University Health Center, IBD Centre, Montréal, Canada; IBD centre McGill University Health Centre
Background
Endoscopic mucosal healing is a key treatment target in inflammatory bowel disease (IBD). The aim of this study was to investigate small bowel (SB) mucosal healing using video capsule endoscopy (VCE) in patients with Crohn’s disease (CD) following 24 weeks of adalimumab therapy.
Methods
In this prospective study, moderate to severe SB involvement CD patients were enrolled, defined by a Lewis score on VCE at diagnosis > 790 in at least one tertile. All patients were treated with adalimumab monotherapy at the standard dosing for CD for 24 weeks prior to undertaking the follow-up second VCE. The primary outcome was the endoscopic healing defined as a Lewis score (LS) < 350, whereas partial response was defined as a > 50% decrease from baseline in LS. Secondary outcomes included the remission in the clinical symptoms (Harvey Bradshaw Index, HBI < 4) and biomarkers (fecal calprotectin, FC < 200 ug/g, and c-reactive protein, CRP < 5 mg/l).
Results
A total of 59 CD patients were screened at McGill University Health Centre between November 2011 and October 2021; 15 patients were met the eligibility criteria and were enrolled. The median age at inclusion was 46 years (IQR 28-51), 8 patients (53.3%) had ankylosing spondylitis. The most frequent locations of CD on VCE findings were the proximal-mid ileum (93.3%) and jejunum (73.3%). Primary outcome; complete endoscopic healing was observed in 8 patients (53.3%, p=0.002, OR for remission = 2.14, 95%CI: 1.25-3.68), and partial response in an additional 5 patients (33.3%) after 24 weeks. The median total Lewis score was decreased significantly compared to the baseline (1,912 vs. 337, p=0.0005). Secondary outcomes; the HBI at baseline was elevated (> 4) in 11 patients. Post-treatment HBI was consistent with clinical remission in all cases (11/15 vs. 0/15, p <0.0001 OR clinical remission = 0.27,95%CI:0.12-0.62). Nine patients who had baseline CRP elevation had achieved normal CRP after treatment, and the median CRP was significantly decreased from 7.3 mg/L (1.8 -18.0) to 1.6 mg/L (IQR 1.0 – 3.2), p=0.025. However, there were no significant changes in FC pre-and post-treatment. In addition, baseline VCE demonstrated ulcerated SB strictures in 3 patients, 2 had non-ulcerated strictures on the second VCE. No capsule retention or other adverse events were observed.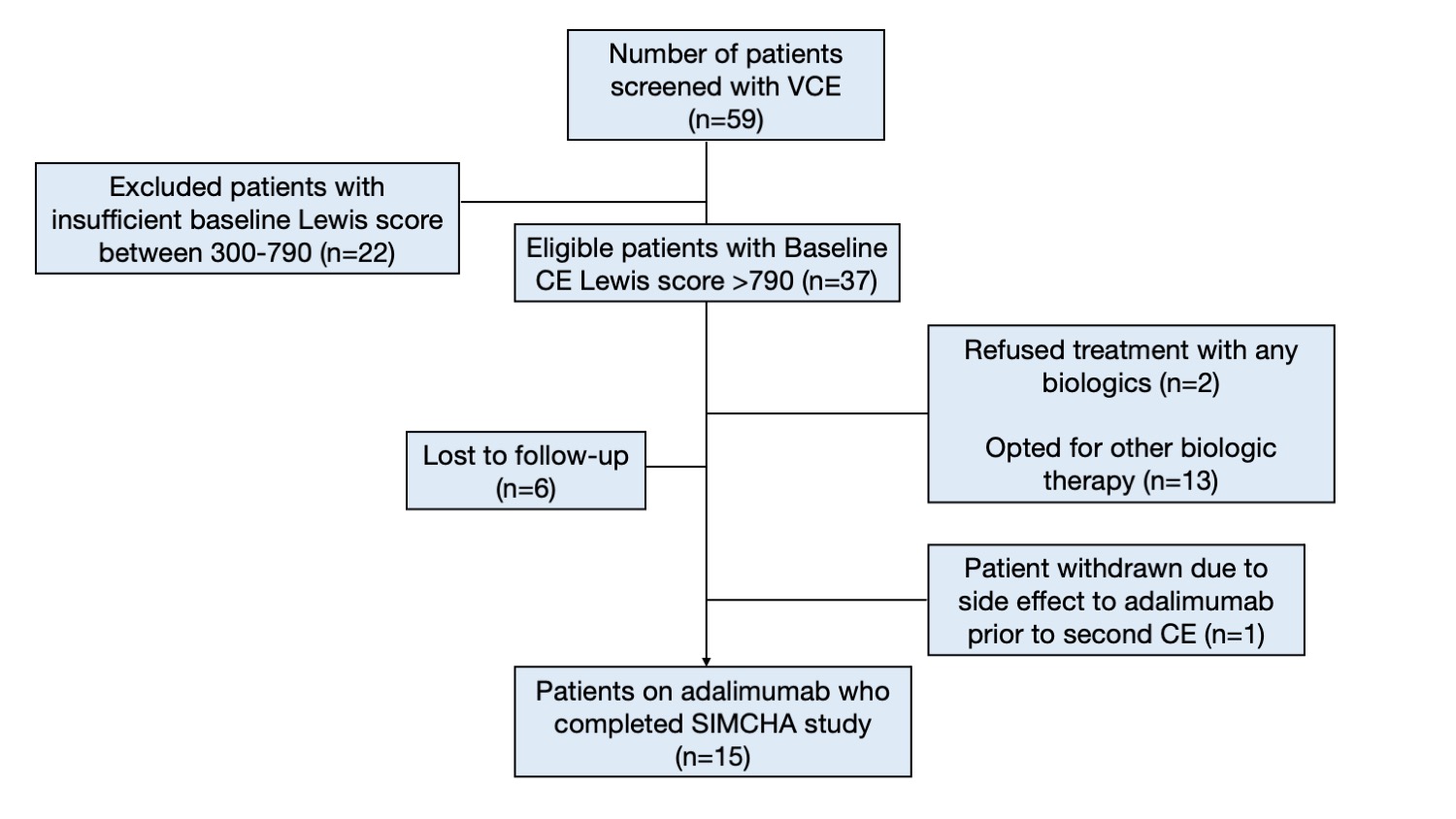



Conclusion
Adalimumab led to significant improvement of small intestinal endoscopic healing in Crohn’s disease, with 53% achieving complete healing.


