P326 The effect of compliance during exclusive enteral nutrition on faecal calprotectin levels in children with Crohn’s disease
Mckirdy, S.(1);Russell, R.(2);Hansen, R.(3);Svolos, V.(1);Gkikas, K.(1);Logan, M.(1);Gerasimidis, K.(1);
(1)University of Glasgow, Human Nutrition, Glasgow, United Kingdom;(2)Royal Hospital for Children and Young People, Department of Paediatric Gastroenterology, Edinburgh, United Kingdom;(3)Royal Hospital for Children, Department of Paediatric Gastroenterology, Glasgow, United Kingdom;
Background
It is yet unclear whether suboptimal response to exclusive enteral nutrition (EEN), in some children with Crohn’s disease (CD), is explained by poor compliance. All proprietary feeds used for EEN are gluten-free; hence patients’ compliance to EEN could be determined by detecting gluten immunogenic peptide (GIP), a biomarker of gluten intake, in faeces.
Methods
The concentration of GIP was measured in the faeces of 45 children (3-17 years) with CD prior to and during (33 & 54 days) treatment with EEN. Associations with GIP and faecal calprotectin (FCAL) levels were explored at 33 and 54 days of EEN.
Results
GIP was present in 37 of the 40 (93%) patients who provided stool samples prior to starting EEN, indicating typical gluten consumption in CD patients. In patients with undetectable GIP at both 33 and 54 days of EEN, FCAL significantly decreased from baseline (mean decrease, 33 days: -743mg/kg, 54 days: -1043mg/kg, p<0.001), but not in patients who had detectable GIP. At EEN completion, patients with undetectable GIP had a lower FCAL by 763mg/kg than patients with a positive GIP result (p=0.041) and demonstrated a greater decline from baseline FCAL (-69% vs +5.%, p=0.021).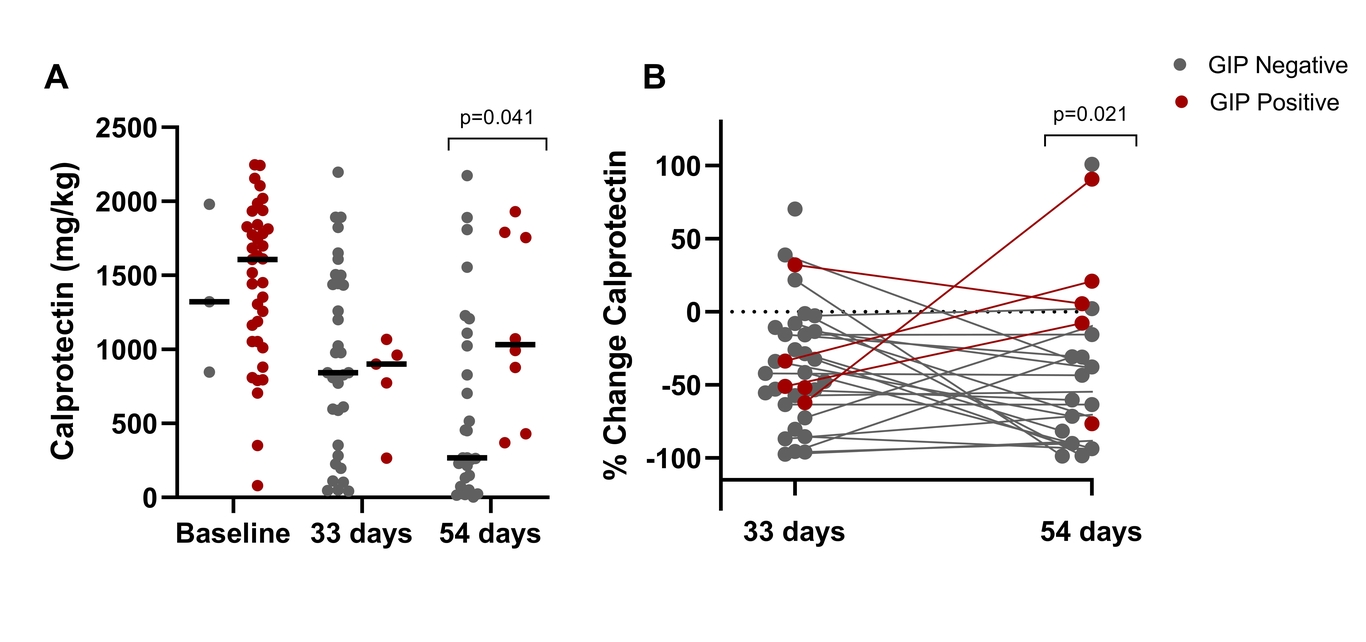
Conclusion
Poor response to EEN might be explained at least in part by diminished compliance and dietary transgressions. Faecal GIP might be useful as a proxy biomarker of EEN compliance.


