P327 Effectiveness of anti-TNF vs. vedolizumab as a second biologic in IBD: results from national Swedish registers
S. Rundquist1, M. Sachs2, C. Eriksson1, O. Olén3, S. Montgomery4, J. Halfvarson1, SWIBREG Study Group
1Department of Gastroenterology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden, 2Clinical Epidemiology Division, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden, 3Clinical Epidemiology Division, Department of Medicine Solna, Department of Clinical Science and Education Södersjukhuset, Karolinska Institutet, Stockholm, Sweden, 4Department of Clinical Epidemiology and Biostatistics, School of Medical Sciences, Örebro University, Örebro, Sweden
Background
We aimed to evaluate the effectiveness of tumour necrosis factor antagonist (anti-TNF) compared with vedolizumab (VDZ) as a second-line biological treatment in IBD.
Methods
A propensity score-matched cohort was created using data from the Swedish National Patient Register, the Swedish Quality Register for IBD (SWIBREG) and the Swedish Prescribed Drug Register. Patients with Crohn’s disease (CD) or ulcerative colitis (UC) exposed to anti-TNF as a first-line biologic, who initiated a second biologic agent 1 May 2014 – 31 December 2016 were included (
Results
After 1:1 propensity score matching, the cohort was restricted to 400 patients (CD,
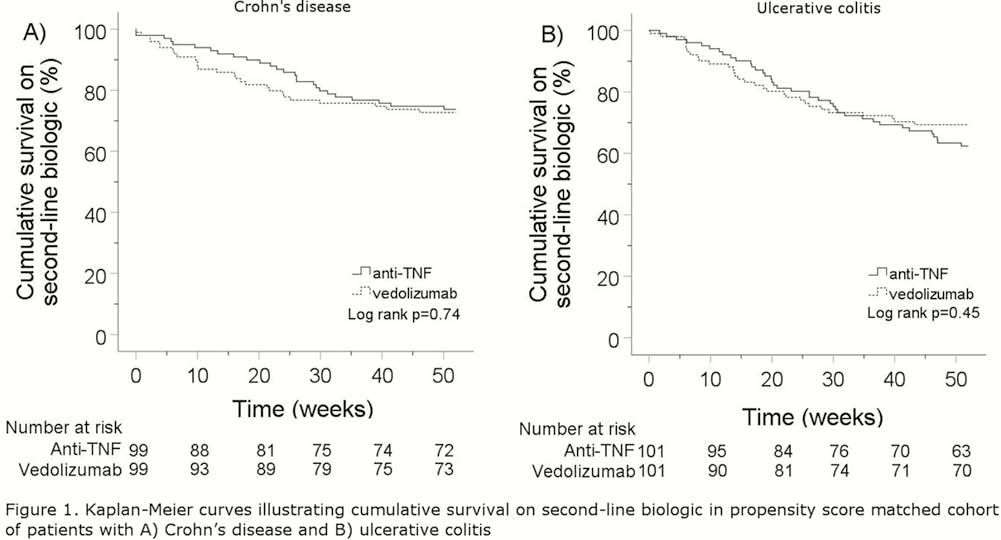
Conclusion
In this propensity score-matched cohort, the effectiveness and safety of anti-TNF and vedolizumab appear comparable when used as a second-line biologic in CD. In patients with UC, risk of hospitalisation and surgery was lower in patients treated with anti-TNF. Randomised controlled trials are needed to confirm these findings.
Support provided by Takeda.


