P328 Ustekinumab is an effective and safe therapy in anti-TNF refractory Crohn’s Disease: a two year real-life observational study from Spain
Rueda Sanchez, J.(1);Cabello Ramirez, M.(1);Camara Baena, S.(1);Keco Huerga, A.(1);Garcia de la Borbolla Serres, J.(1);Castro Fernandez, M.(1);Grande Santamaria, L.(1);
(1)Virgen de Valme Universitary Hospital, CGU Gastroenterology, Sevilla, Spain
Background
Ustekinumab is a monoclonal antibody targeting IL-12/23 and was proved efficacious during the registration clinical trials. However real-world data in a day to day setting is still scarce. The aim of our study was to evaluate the effectiveness, durability and safety of ustekinumab in our real-life cohort.
Methods
We present a retrospective, observational study from a single tertiary center. We included adult CD patients that had received the standard ustekinumab intravenous induction and with at least 12-month follow-up. We assessed clinical (HBI) and biomarker (CRP, calprotectin) response at 3, 6, 12, 18 and 24 months. Adverse events, perianal disease response and corticosteroid (CS) use were also recorded.
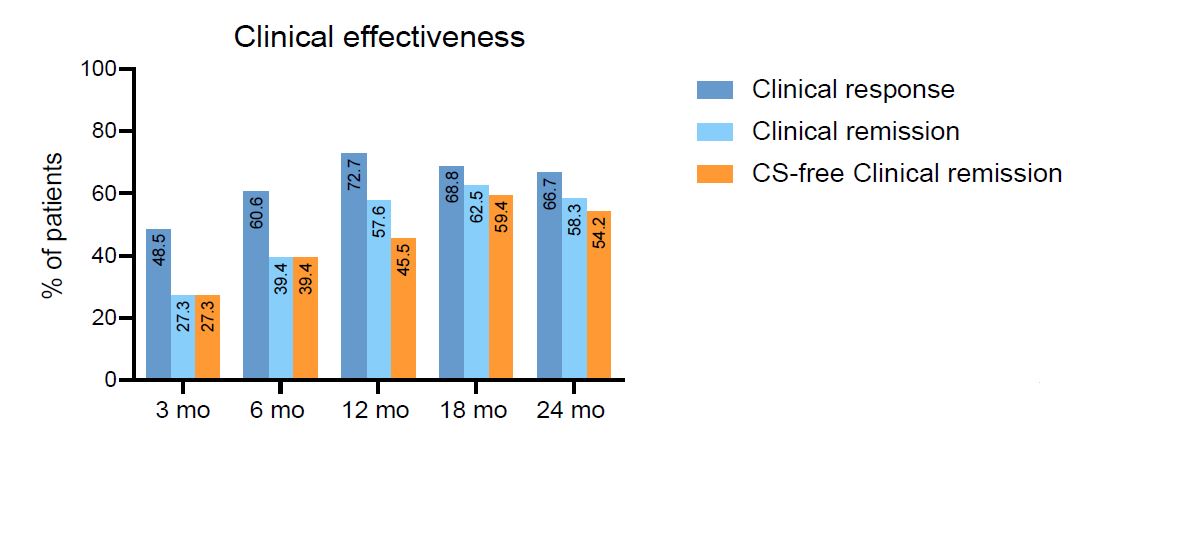
Results
We observed a significant decrease in median HBI in all visits. Clinical response was observed in 72.7% of patients at 12 months and 66.7% at 24 months. Clinical remission was achieved in 57.6% and 58.3% of patients at 12 and 24 months respectively. CS-free clinical remission rates were 45.5% at 12 months and 54.2% at 24 months. The most frequent maintenance dose interval was q8w, with only 6/35 patients (17,14%) requiring dose escalation due to inefficacy of standard q8w interval. Drug survival at 2 years was 93.9%. Perianal disease clinical improvement was noted in 16 out of 17 patients with perianal disease at baseline. Fecal calprotectin decreased significantly from baseline, with a median change of -66 ug/g at 12 months and -253 ug/g at 24 months.
Ustekinumab was generally well-tolerated. Two adverse events were recorded during the follow-up period, an herpes zoster and an uveitis, none of them required Ustekinumab discontinuation.
Conclusion
According to our results, Ustekinumab was effective, durable, and safe for moderate-severe CD in a real-life clinical setting, with more than half of patients achieving CS-free clinical remission at 24 months in an anti-TNF refractory cohort.
| N = 35 | |
| Age (Median, IQR) | 44 (33-55) |
| Sex (M/F); n,% | 15(43%) / 20 (57%) |
| Age at diagnosis; n,% - 17-40 y (A2) - >40 y (A3) | 13 (37,14%) 22 (62,85%) |
| Disease location; n,% - Ileal (L1) - Colonic (L2) - Ileocolonic (L3) - Upper digestive (L4) | 5 (14%) 4 (11,43%) 26 (74,29%) 2 (5,71%) |
| Disease phenotype; n,% - Inflammatory (B1) - Stricturing (B2) - Penetrating (B3) - Perianal | 15 (42,86%) 11 (31,43%) 9 (25,71%) 17 (48.57%) |
| Intestinal resection; n,% | 8 (22,86%) |
| Clinical activity - H. Bradshaw Index; median, IQR - CDAI; median, IQR | 8 (6-10) 217 (171-247) |
| Laboratory markers - CRP (mg/l); median,IQR - Calprotectin (mg/Kg); median,IQR | 5.3 (2-13.4) 670 (232-1336) |
| Previous treatment - Corticosteroids - Immunomodulators - AntiTNF | 31 (88,57%) 32 (91,42%) 33 (94,28%) * ≥2 AntiTNF: 24 (68,57%) |