P333 Biologic longevity: Withdrawal of drugs due to remission is rare in clinical practice
E. Sharma, G. Cunningham, P. Dawson, F. D’Errico, S. Honap, S. Lim, R. Luber, S. Meade, P. Pavlidis, R. Reynolds, P. Irving
Guy’s and St Thomas’ NHS Foundation Trust, St Thomas Hospital, London, UK
Background
Biological medicines are well established in the treatment of inflammatory bowel disease (IBD). The aim of this study was to review the natural history of biologic use in a large cohort of patients from a tertiary centre.
Methods
We performed a retrospective review of prospectively collected records of patients with Ulcerative colitis (UC) and Crohn’s disease (CD) from a single centre. All patients who received ustekinumab, golimumab and vedolizumab between April 2014 and May 2019 were included as were a similar sized cohort of patients who received adalimumab and infliximab. Demographic data, use of concomitant immunomodulation, length of time on drug and reason for stopping treatment were recorded.
Results
The patient cohort is described in Table 1. Median (range) follow-up was 32 (3–61) months. Infliximab and golimumab were used first line in 92 (94%) patients and 83 (87%) patients, respectively. Ustekinumab and vedolizumab were used later on in treatment with 38 patients receiving ustekinumab third line (47%) and vedolizumab being used second line in 52 patients (44%) and third line in 43 (37%) patients. Table 2 describes the reasons for biologic cessation. Other reasons for discontinuation included adverse drug reaction, pregnancy and patient choice. Concomitant immunomodulation at initiation of biologic was seen in 228 (79%) patients on TNF antagonists, 58 (50%) patients on vedolizumab and 44 (54%) patients on ustekinumab.
| Biologic | All | CD | UC | IBDU |
| Adalimumab | 95 | 93 | 2 | |
| Golimumab | 95 | 95 | ||
| Infliximab | 98 | 95 | 1 | 2 |
| Ustekinumab | 81 | 78 | 3 | |
| Vedolizumab | 117 | 66 | 43 | 8 |
| Total | 486 | 332 | 141 | 13 |
| Adalimumab | Golimumab | Infliximab | Ustekinumab | Vedolizumab | |
| Median time on drug (months) | 75 | 12 | 74 | 12 | 26 |
| Number of patients stopping, n (%) | 35 (37) | 51 (54) | 49 (50) | 10 (12) | 54 (46) |
| Reasons for stopping, n (%) | |||||
| Remission | 10 (29) | 4 (8) | 17 (35) | 0 (0) | 2 (4) |
| Active disease | 20 (57) | 44 (86) | 23 (47) | 8 (80) | 39 (72) |
| Other | 5 (14) | 3 (6) | 9 (18) | 2 (20) | 14 (26) |
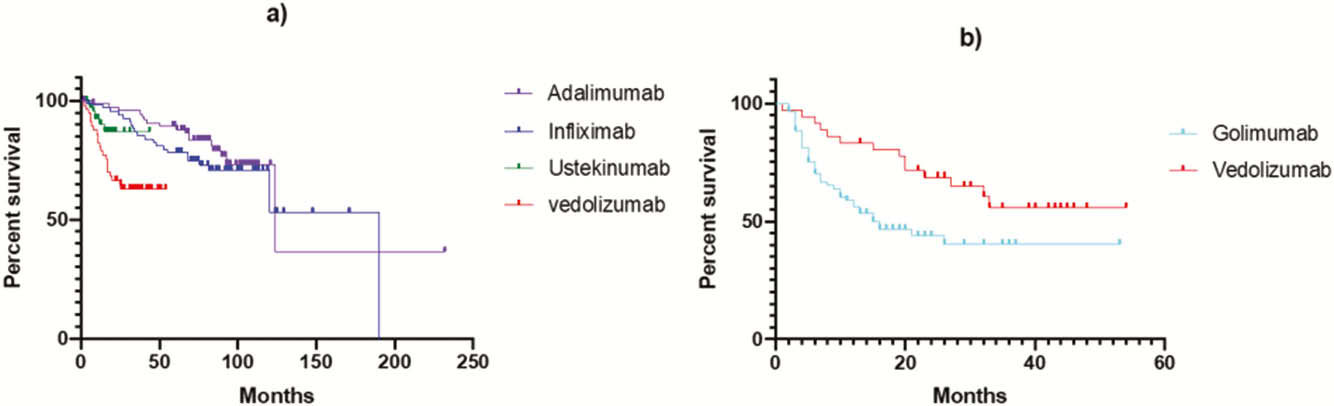
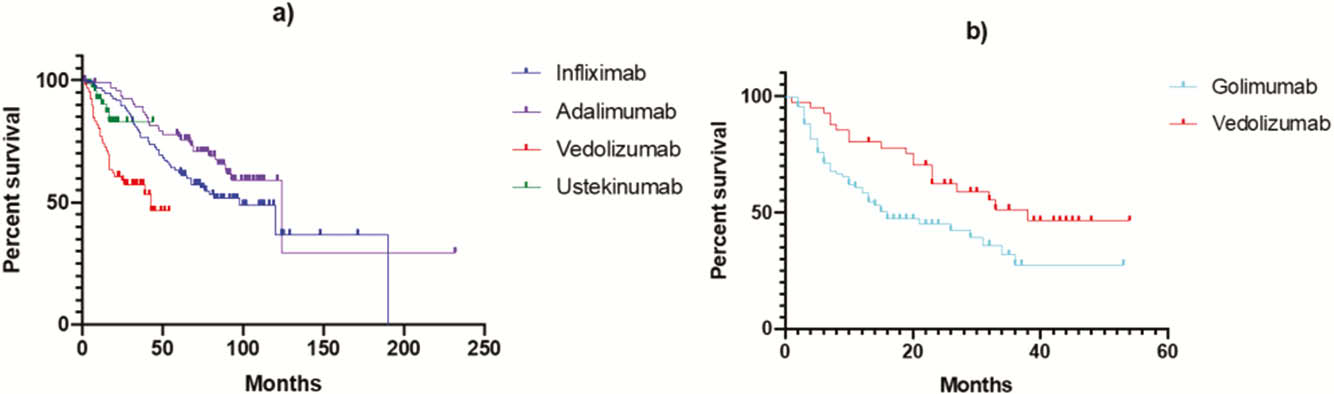
Conclusion
In CD, adalimumab and infliximab display a longer drug survival compared with ustekinumab and vedolizumab possibly due to treatment positioning. In UC, vedolizumab is associated with more treatment persistence than golimumab. Across all biologics, documented cessation due to remission was low, especially in those biologics that are used second or third line.


