P333 Precise and unbiased infliximab dosing in patients with inflammatory bowel diseases using a multi-model averaging approach
Kantasiripitak, W.(1);Outtier, A.(2,3);Thomas, D.(1);Kensert, A.(1);Wang, Z.(1);Sabino, J.(2,3);Wicha, S.G.(4);Vermeire, S.(2,3);Ferrante, M.(2,3);Dreesen, E.(1);
(1)University of Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium;(2)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(3)University of Leuven, Department of Chronic Diseases and Metabolism, Leuven, Belgium;(4)University of Hamburg, Department of Clinical Pharmacy, Hamburg, Germany;
Background
Underexposure to IFX is a common cause of loss of response in patients with inflammatory bowel disease (IBD). To ensure adequate – but not unnecessarily high – exposure, we aimed to identify a precise and unbiased approach for model-based dosing of IFX in patients with IBD.
Methods
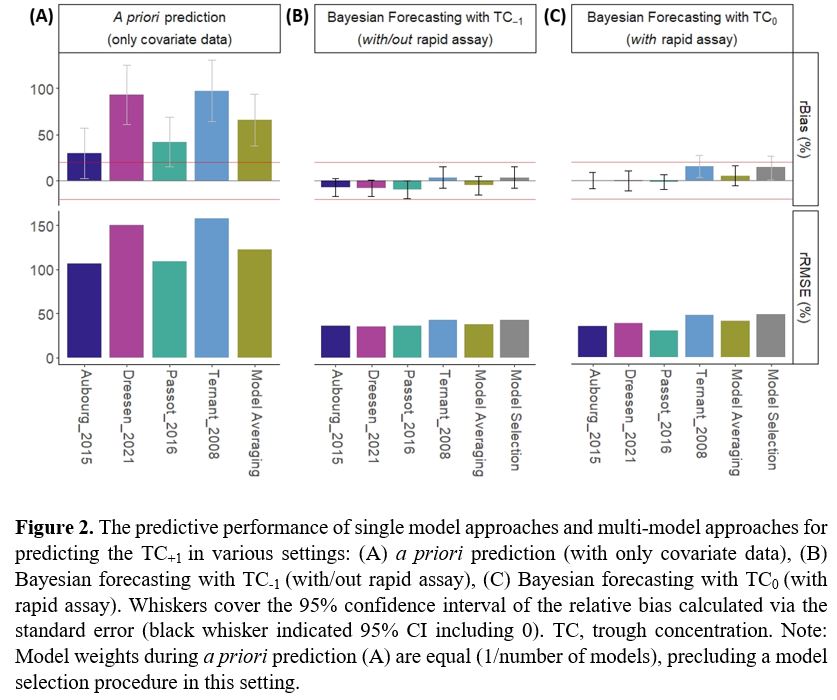
A retrospective study was performed using data from 54 patients on IFX maintenance therapy. The predictive performance of 18 published IFX population pharmacokinetic (popPK) models was evaluated using NONMEM (v7.5). A priori prediction (only based on covariates) and Bayesian forecasting (BF; also based on one to three consecutively measured IFX trough concentrations; TC-2, TC-1, and TC0) of the IFX TC+1 was evaluated (Fig 1). The predictive performance of a single-model approach was compared with two automated multi-model approaches: a model selection algorithm (MSA) and a model averaging algorithm (MAA).1 Relative bias (rBias) and relative root mean square error (rRMSE) were used to determine bias and imprecision of the predicted versus observed TC+1. Clinical acceptability was defined as an rBias between ±20% with a 95%CI including zero. The predicted and observed TC+1 were classified at 5.0 mg/L TC target.2
Results
Four models were selected based on their predictive performances and implemented in the TDMx software tool to support model-based IFX dosing in the forthcoming prospective MODIFI study (NCT04982172).
A priori prediction of TC+1 was clinically unacceptable with both single- and multi-model approaches (rBias +30% to +97%, rRMSE 107% to 158%; Fig 2A). Also, a priori prediction had the lowest classification accuracy (median 59%, IQR 59%-63%; Fig 3A). Providing one IFX TC greatly improved predictive performance (rBias -10% to +15%, rRMSE 30% to 49%; Fig 2B, 2C) and classification accuracy (TC-1: median 70%, IQR 63%-72%; TC0: median 80%, IQR 76%-84 %; Fig 3B, 3C). More specifically, BF resulted in a significantly lower chance of a falsely predicted ≥5 mg/L TC+1 than a priori prediction (p<0.01). In comparison with BF with TC-1, the availability of TC0 significantly lowered the chance of falsely predicting TC+1 <5 mg/L (unnecessary dose optimisation) (p=0.0034).
Providing more than one previous TC improved predictive performances only marginally (data not shown). In general, MAA performed better than MSA.

Conclusion
A multi-model averaging approach provided more reliable Bayesian forecasts than the single-model approach. Adding one previous trough concentration in addition to covariate information sufficed to provide accurate and unbiased predictions of future exposure. Concentration data collected with a rapid assay may reduce the chance of performing unnecessary dose optimisation.
Ref 1Uster D CPT,2021;2Vande Casteele N Gastroenterology,2017



