P335 Real-life effectiveness and safety of tofacitinib treatment in patients with ulcerative colitis: A KASID multicenter cohort study
Oh, K.(1);Hong, S.N.(2);Kim, E.S.(3);Na, S.Y.(4);Kang, S.B.(5);Koh, S.J.(6);Bang, K.B.(7);Jung, S.A.(8);Kim, K.O.(9);Choi, C.H.(10);Ye, B.D.(11);
(1)University of Ulsan College of Medicine- Asan Medical Center, Gastroenterology, Seoul, Korea- Republic Of;(2)Samsung Medical Center- Sungkyunkwan University School of Medicine, Medicine, Seoul, Korea- Republic Of;(3)Kyungpook National University School of Medicine, Internal Medicine, Daegu, Korea- Republic Of;(4)College of Medicine- Incheon St. Mary's Hospital- The Catholic University of Korea, Internal Medicine, Incheon, Korea- Republic Of;(5)College of Medicine- Daejeon St. Mary's Hospital- The Catholic University of Korea, Internal Medicine, Daejeon, Korea- Republic Of;(6)Seoul National University College of Medicine, Internal Medicine and Liver Research Institute, Seoul, Korea- Republic Of;(7)Dankook University College of Medicine, Internal Medicine, Cheonan, Korea- Republic Of;(8)Ewha Womans University School of Medicine, Internal Medicine, Seoul, Korea- Republic Of;(9)Yeungnam University College of Medicine, Internal Medicine, Daegu, Korea- Republic Of;(10)Chung-Ang University College of Medicine, Internal Medicine, Seoul, Korea- Republic Of;(11)University of Ulsan College of Medicine- Asan Medical Center, Gastroenterology and Inflammatory Bowel Disease Center, Seoul, Korea- Republic Of; IBD Research Group of the Korean Association for the Study of Intestinal Diseases
Background
Tofacitinib is a small molecule which inhibits janus kinase and has been shown to be effective for patients with active ulcerative colitis (UC). We investigated the real-life therapeutic effectiveness and safety of tofacitinib treatment for Korean patient with UC.
Methods
We retrospectively analyzed the data of patients with UC who received tofacitinib treatment at 10 hospitals in Korea. The primary outcome was clinical remission at week 16 which was defined as a partial Mayo score ≤2 with a combined rectal bleeding subscore and stool frequency subscore ≤1. The secondary outcome was endoscopic remission (Mayo endoscopic subscore ≤1) at week 16. Adverse events including herpes zoster and deep vein thrombosis were also evaluated.
Results
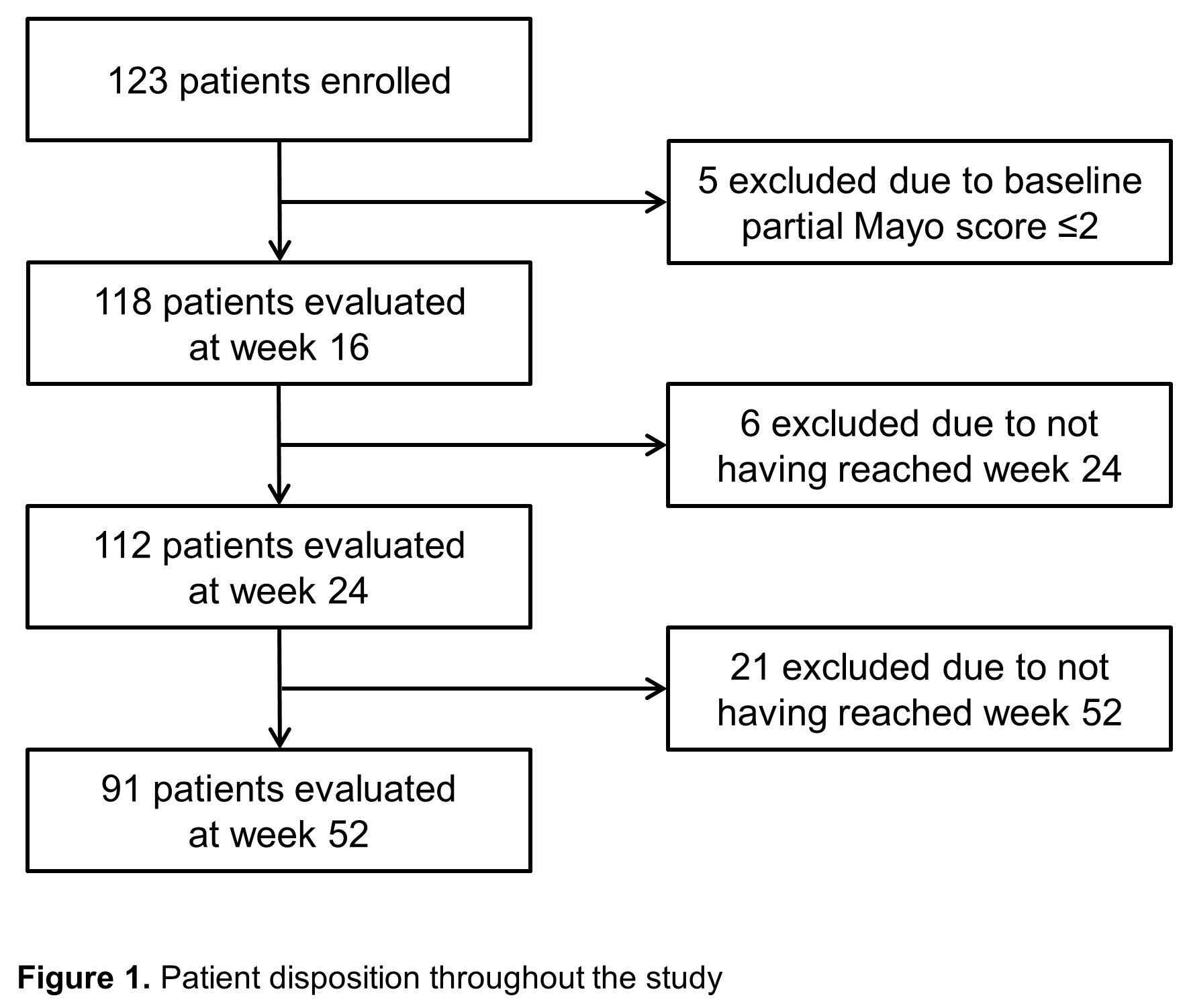
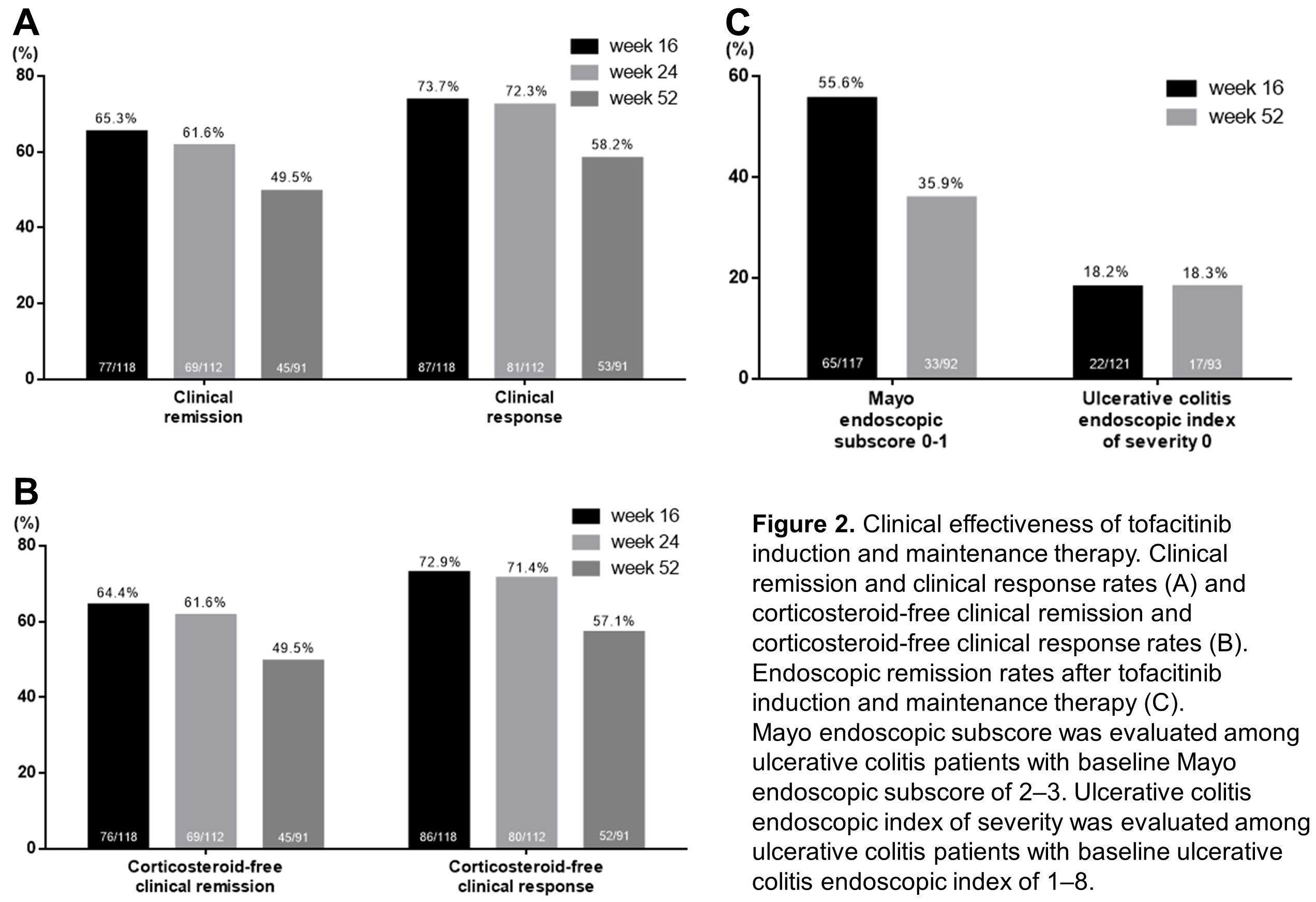
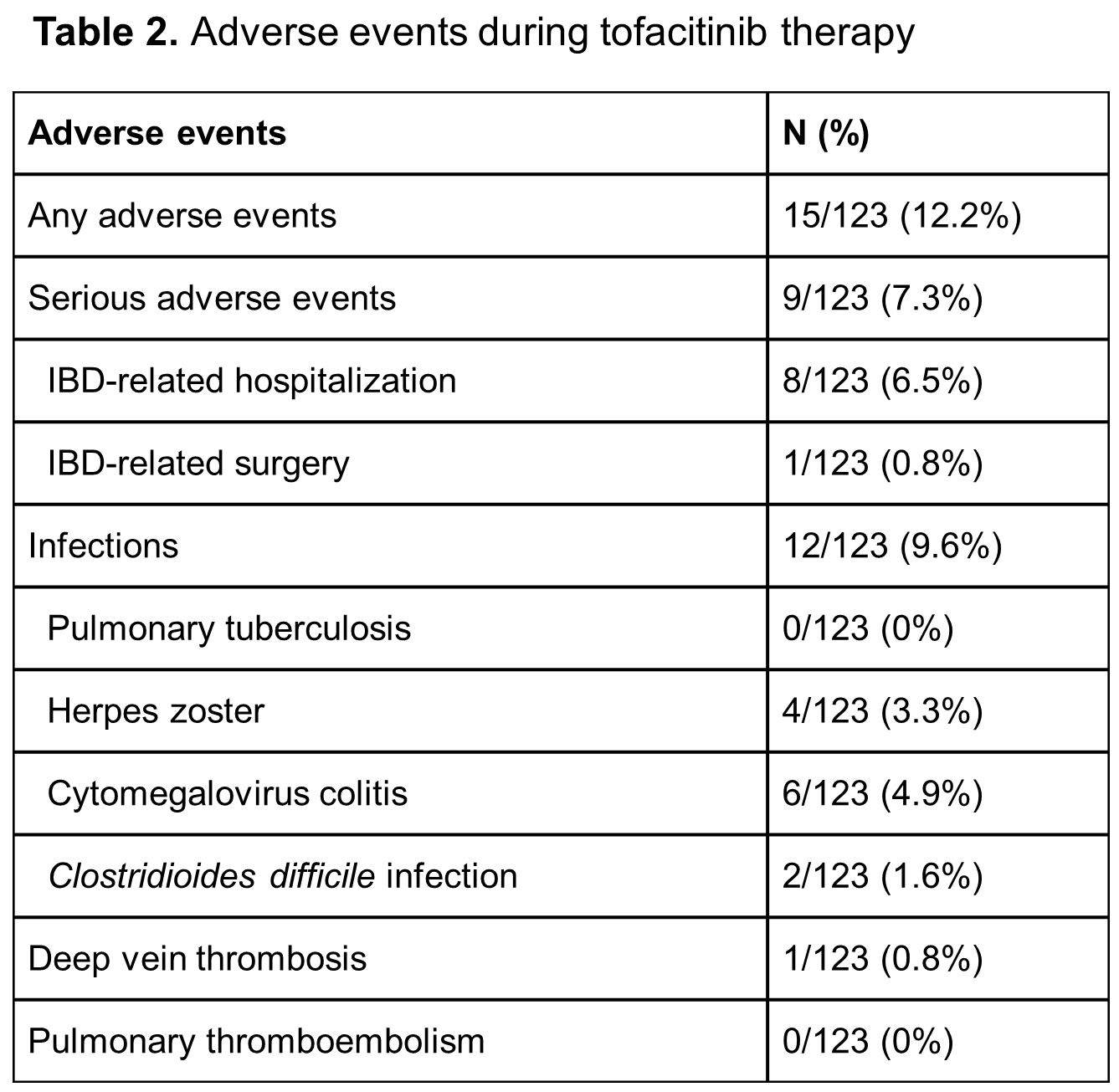
Between January 2018 and November 2020, a total of 123 patients with UC received tofacitinib treatment (Table 1). Clinical effectiveness was evaluated for those who had completed or stopped the tofacitinib therapy by week 16, week 24 and week 52, respectively (Figure 1). Clinical remission and response rates at week 16 were 65.3% and 73.7%, respectively (Figure 2A). Ninety-one out of 123 patients (74.0%) were followed up to week 52. Their clinical remission and response rates at week 52 were 49.5% and 58.2%, respectively (Figure 2A). Among 117 patients with baseline Mayo endoscopic subscore ≥2, endoscopic remission rate at week 16 was 55.6% (Figure 2C). Any adverse events occurred in 15 patients (12.2%) and serious adverse events occurred in 9 patients (7.3%). Herpes zoster occurred in 4 patients (3.3%) and one patient (0.8%) suffered from deep vein thrombosis (Table 2).
Conclusion
Tofacitinib was effective with an acceptable safety profile for Korean patients with UC in the real world.