P337 Usefulness of drug-level monitoring in therapeutic guidance in patients with ulcerative colitis on anti-TNF-α maintenance therapy
E.H. Oh1, Y. A-Ran2, K. Ye-Jee3, K. Jeongseok1, N. Namseok1, P. Sang Hyoung4, Y. Dong-Hoon1, B. Jeong-Sik1, M. Seung-Jae1, Y. Suk-Kyun4, Y. Byong Duk4
1Asan Medical Center, University of Ulsan College of Medicine, Department of Gastroenterology, Seoul, Korea Republic of, 2Asan Medical Center, University of Ulsan College of Medicine, Inflammatory Bowel Disease Center, Seoul, Korea Republic of, 3Asan Medical Center, University of Ulsan College of Medicine, Department of Clinical Epidemiology and Biostatistics, Seoul, Korea Republic of, 4Asan Medical Center, University of Ulsan College of Medicine, Department of Gastroenterology and Inflammatory Bowel Disease Center, Seoul, Korea Republic of
Background
While treatment optimisation using therapeutic drug monitoring (TDM) of anti-TNF-α agents has become more common in clinical practices, studies comparing its correlation with clinical remission (CR), biochemical remission (BR) and mucosal healing (MH) in patients with ulcerative colitis (UC) are still lacking
Methods
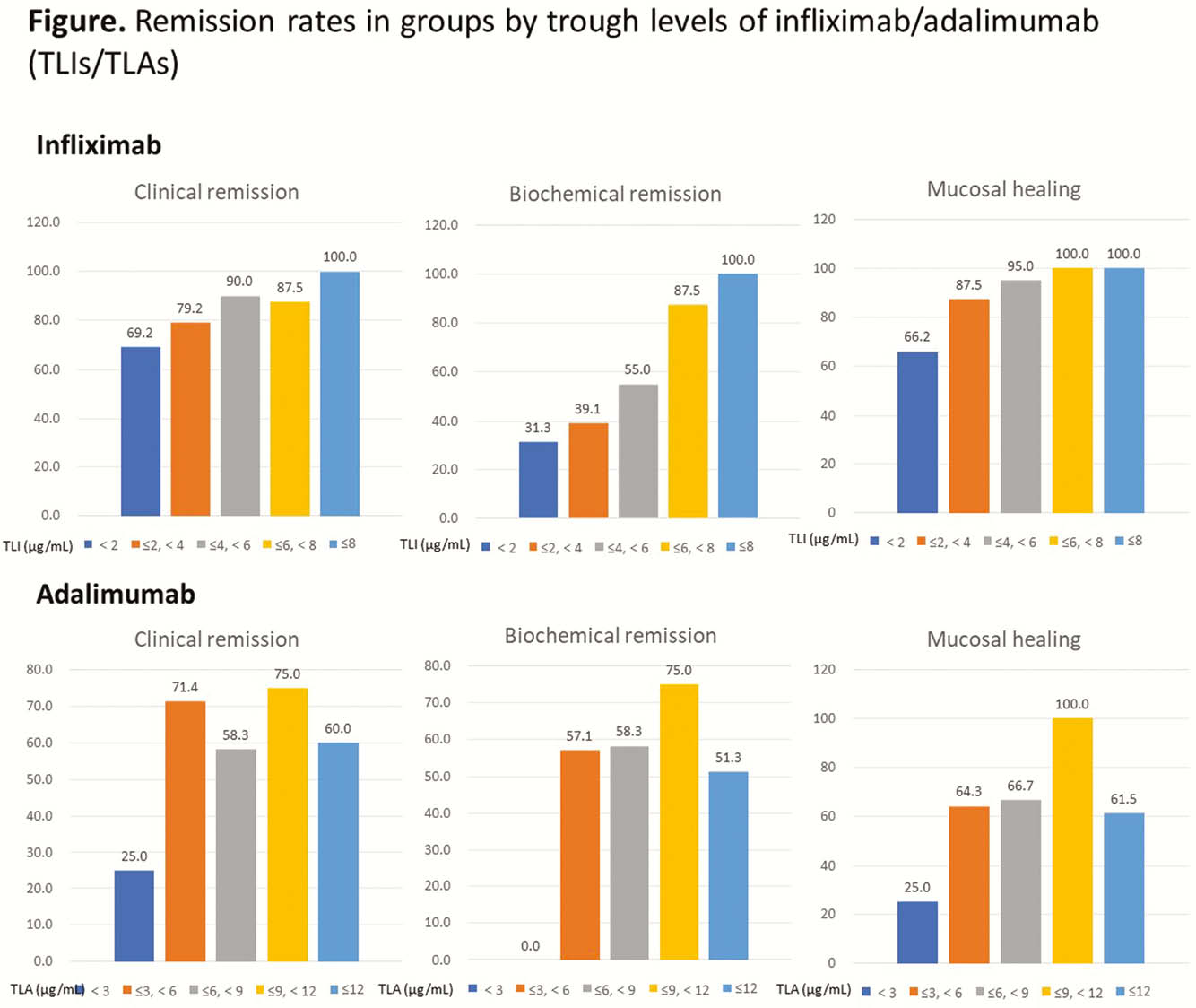
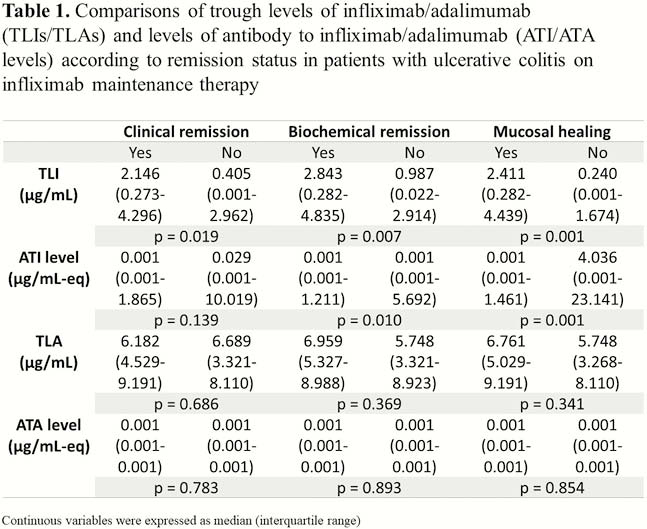
Trough levels of infliximab/adalimumab (TLIs/TLAs) and levels of antibody to infliximab/adalimumab (ATI/ATA levels) were measured using serum samples collected between August 2017 and September 2019 from patients with UC on anti-TNF-α maintenance therapy. CR, BR and MH were defined as partial Mayo score of 0–1, within normal range of both ESR and serum C-reactive protein (CRP) level, and Mayo endoscopic subscore of 0–1, respectively.
Results
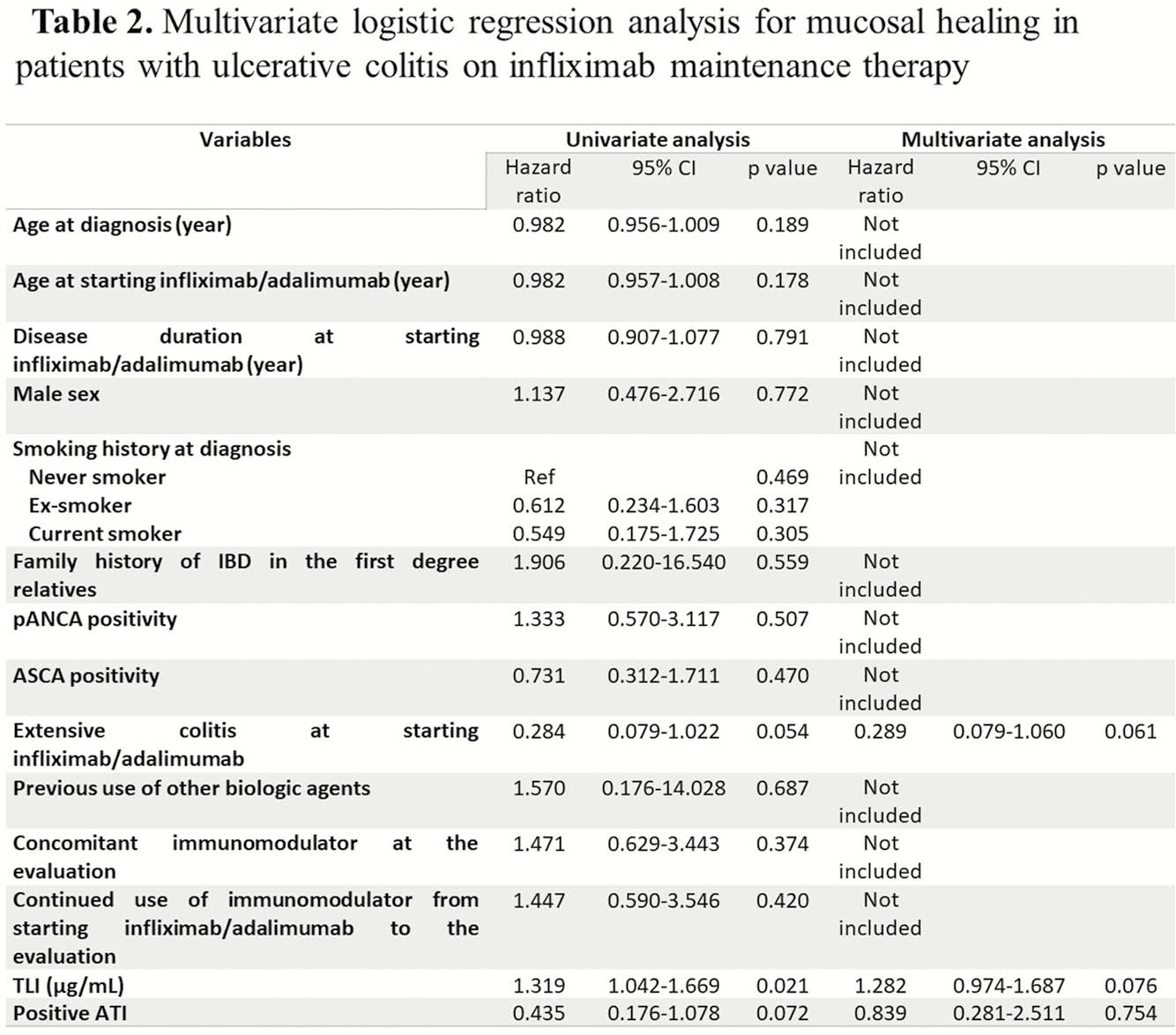
This study included 115 patients (72.8%, 91 [76.5%] on infliximab and 24 [61.5%] on adalimumab) in CR and 43 patients (27.2%) in non-CR. ATI/ATA were positive in 31 (26.1%) and 2 (5.1%) patients on infliximab/adalimumab therapy, respectively. TLI showed significant differences according to stats of CR (2.146 [0.273–4.296]
Conclusion
In this study, TLIs correlated with CR, BR and MH, while TLAs did not. TLI should be targeted to be above 2.3 μg/ml.


