P340 Comorbidity is associated with safety outcomes in vedolizumab- and ustekinumab-treated inflammatory bowel disease patients
V. Asscher1, V. Biemans2,3, M. Pierik3, G. Dijkstra4, A. Van der Meulen-de Jong1, M. Löwenberg5, S. van der Marel6, N. de Boer7, A. Bodelier8, J. Jansen9, R. West10, J. Haans3, W. van Dop2, R. Weersma4, F. Hoentjen2, J. Maljaars1, on behalf of the Dutch Initiative on Crohn and Colitis (ICC)
1Leiden University Medical Center, Department of Gastroenterology and Hepatology, Leiden, The Netherlands, 2Radboud University Medical Centre, Department of Gastroenterology and Hepatology, Nijmegen, The Netherlands, 3Maastricht University Medical Centre, Department of Gastroenterology and Hepatology, Maastricht, The Netherlands, 4University Medical Centre Groningen, Department of Gastroenterology and Hepatology, Groningen, The Netherlands, 5Amsterdam University Medical Centre- Academic Medical Centre, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands, 6Haaglanden Medical Centre, Department of Gastroenterology and Hepatology, Den Haag, The Netherlands, 7Amsterdam University Medical Centre- Vrije Universiteit Amsterdam, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands, 8Amphia Hospital, Department of Gastroenterology and Hepatology, Breda, The Netherlands, 9Onze Lieve Vrouwe Gasthuis, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands, 10Franciscus Gasthuis and Vlietland, Department of Gastroenterology and Hepatology, Rotterdam, The Netherlands
Background
The effect of comorbidity on treatment outcomes of vedolizumab and ustekinumab in inflammatory bowel disease (IBD) patients is currently unknown. We aimed to assess the impact of comorbidity on safety and effectiveness of therapy in vedolizumab and ustekinumab treated IBD patients.
Methods
In this multi-centre prospective
Results
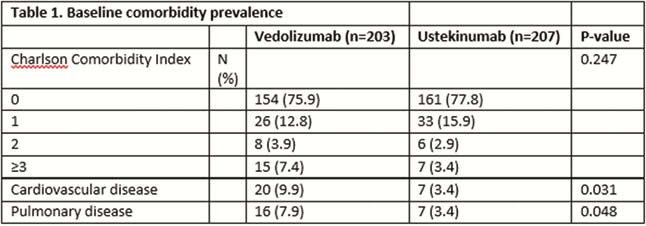
We included 203 vedolizumab and 207 ustekinumab treated patients: mean age 42.2 (SD 16.0) and 41.6 (SD 14.4) years, median HBI 7.0 (IQR 5.0–10.0) or SSCAI 5.0 (IQR 3.0–8.0) and median HBI 7.0 (5.0–11.8). Median treatment duration 54.0 (IQR 19.9–104.0) and 48.4 (IQR 24.4–55.1) weeks, median follow-up time 104.0 (IQR 103.1–104.0) and 52.0 weeks (IQR 49.3–100.4), respectively. More cardiovascular disease (congestive heart failure, myocardial infarction, peripheral vascular disease and cerebrovascular accidents/transient ischaemic attacks) and more pulmonary disease were present in vedolizumab patients at baseline (Table 1). CCI associated independently with safety outcomes in vedolizumab and ustekinumab. In vedolizumab, CCI associated with infection (OR 1.387, 95% CI 1.022–1.883,
Conclusion
Comorbidity associated with all-cause hospitalisation in vedolizumab and ustekinumab treated IBD patients. A significant impact on infections was found only in vedolizumab treated patients. This study underlines the importance of comorbidity assessment in these IBD patients.


