P340 Risankizumab in Crohn's disease: Real World Experience from a Pre-Approval Access Program
Dawson, P.(1);Sharma, E.(1);Dart, R.J.(1);Lim, S.(1);Anderson, S.H.(1);Ray, S.(1);Mawdsley, J.(1);Irving, P.M.(1);Samaan, M.A.(1);
(1)Guys and St Thomas NHS trust, Department of Gastroenterology, London, United Kingdom;
Background
Risankizumab is a monoclonal antibody which inhibits interleukin-23, a key cytokine in the pathogenesis of IBD. It has applications in several immune mediated inflammatory diseases and has recently demonstrated superiority versus placebo as indication and maintenance treatment for moderate-to-severe Crohn's disease (CD). A pre-approval access program (PAAP) granted use for a cohort of patients who had active disease, a previous inadequate response to all licenced biologic therapies for CD and who were ineligible for a clinical trial. We aimed to define the effectiveness and tolerability of risankizumab in this real-world, treatment refractory cohort.
Methods
We performed a retrospective cohort analysis of prospectively maintained data for patients starting risankizumab between January and September 2021. All patients received doses at four weekly intervals. Clinical disease activity, biochemical activity and quality of life were measured using HBI, CRP and IBD-C scores, respectively. Study evaluations were made at baseline, weeks 4 and 12. Data were analysed in GraphPad prism and are described as median (range).
Results
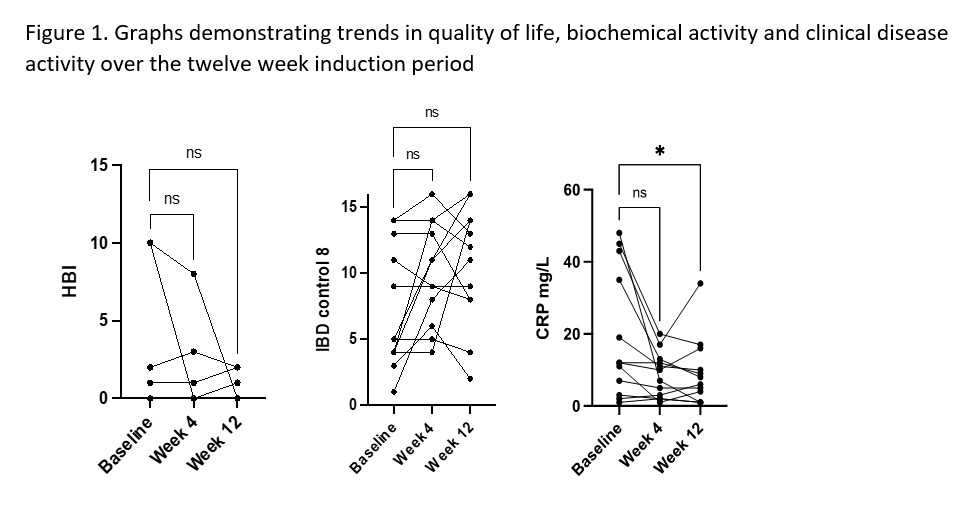
Since January 2021, 16 patients have started Risankizumab via the PAAP. We included 12 patients who had completed the 12 week induction period at the time of submission. Demographics are shown in Table 1. The characteristics are consistent with a more aggressive phenotype with the majority of patients (9 (75%)) having perianal involvement as well as having either stricturing (5(42%)) or internal penetrating disease (3(25%)). Nine (75%) patients had undergone prior luminal surgery, 7 of whom still had stomas, therefore were not included in the analysis for HBI. Median HBI at baseline was 2 (0-10) vs 1 (0-8) at week 4 and 1 (0-2) at week 12 and due to small patient numbers was not statistically significant. There was an improvement in IBD-C scores, although not statistically significant. Median IBD-C score at baseline was 5 (0-14) vs 10 (4-16) at week 4 and 12 (2-16) at week 12. CRP decreased from 12 mg/L (1-48) at baseline to 9 mg/L (1-20) at week 4 (p=0.13) and to 7 mg/L (1-34) at week 12 (p=0.029). Results are represented in Figure 1. Two patients experienced mild headaches, no other side effects were reported. At baseline two patients were being treated with concomitant steroids; one discontinued following induction and one has remained on steroids. No other patients initiated steroids. No patients discontinued risankizumab during the study period.
Conclusion
We present a heterogeneous cohort of patients with refractory CD despite multiple biologic mechanisms. Overall, there was a positive impact of risankizumab induction over a 12 week period with respect to symptoms, quality of life and CRP, showing the effectiveness of risankizumab in this refractory cohort. We will follow up, and at week 24 will objectively assess disease activity with cross sectional imaging and/or endoscopy.


