P346 Single-centre experience of ustekinumab: Therapeutic drug monitoring in Crohn’s disease patients
C. Liefferinckx1,2, M. Fassin2, D. Thomas3, C. Minsart1,2, A. Cremer1,2, L. Amininejad1, V. Tafciu2, V. Wambacq1, A. Van Gossum1, D. Franchimont1,2
1Department of Gastroenterology, Hôpital Erasme, Brussels, Belgium, 2Laboratory of Experimental Gastroenterology, ULB, Brussels, Belgium, 3Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
Background
Therapeutic drug monitoring (TDM) is a diagnostic tool in the monitoring of anti-TNF therapies. Yet, the benefit for TDM of new biologics such as ustekinumab (USK) is still controversial in real-world experiences.
Methods
This monocentric retrospective study aims to correlate USK trough levels (TLs) with clinical and endoscopic data. All patients have given written consent to the Biobank (B2011/005). Endoscopic disease was defined as quiescent in absence of endoscopic lesions, mild disease in presence of few superficial ulcerations, moderate in presence of several ulcers and severe in presence of numerous deep ulcers and/or inflammatory stenosis. 313 serum USK samples from 67 Crohn’s disease patients were used to measure USK TL (USK ELISA, apDia) while 88 samples (at week 16, and before and after optimisation) were used to measure anti-drug antibody (ADA), using a drug-tolerant affinity capture elution anti-ustekinumab assay
Results
Demographic and baseline data of our population are presented in Table 1.
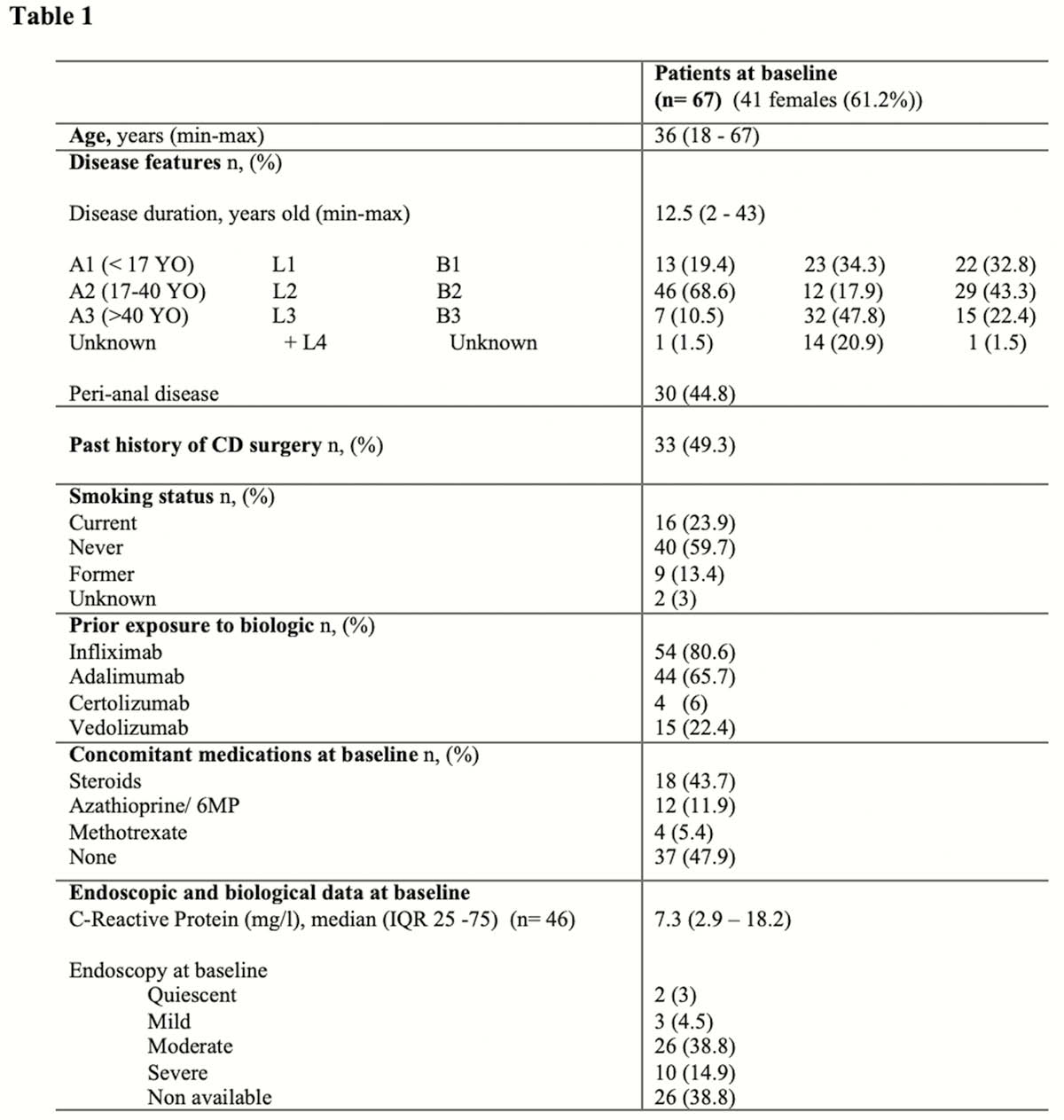
The median follow-up was 73 weeks (IQR 39–92). An optimisation due to loss of response was required in 44.8% of patients (
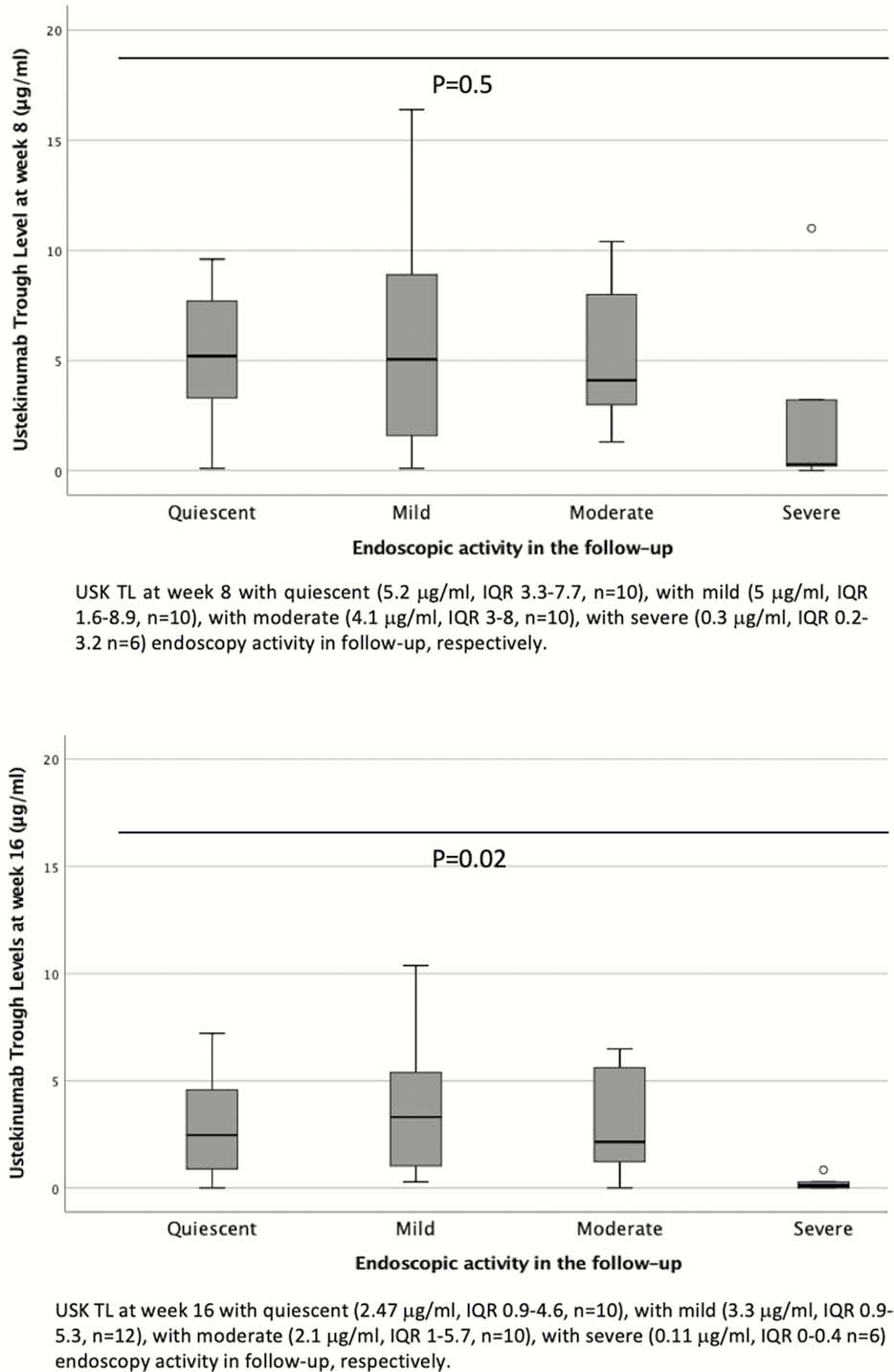
Patients not requiring an optimisation had higher TLs in maintenance than patients requiring optimisation (2.45 µg/ml (IQR 1.3–4.4) vs. 1.15 µg/ml (IQR 0.1–2.24),
Conclusion
This real-world experience confirms a drug exposure-endoscopic response relationship. Week 16 seems to be an appropriate time point to monitor drug exposure. Earlier USK TLs, at week 8, appear less valuable to be monitored due to the influence of initial IV dose. The absence of immunogenicity suggests that it is not a key driver in the loss of response.


