P362 Prospective study of phArmaCokinetics of InFliximab during induction in patients with Crohn’s disease and ulcerative colitis (PACIFIC)
C. Liefferinckx1, P. Bossuyt2, D. Thomas3, J.F. Rahier4, E. Louis5, F. Baert6, P. Dewint7,8, L. Pouillon2, G. Lambrecht9, S. Vermeire10, D. Franchimont1, of Belgian Inflammatory Bowel Disease Research and Development (BIRD)
1Department of Gastroenterology, Hôpital Erasme, Brussels, Belgium, 2Department of Gastroenterology, Imelda General Hospital, Bonheiden, Belgium, 3Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium, 4Department of Gastroenterology, CHU UCL Namur, Yvoir, Belgium, 5Department of Gastroenterology, Centre Hospitalier Universitaire Sart-Tilman, Liège, Belgium, 6Department of Gastroenterology, AZ Delta, Roeselare, Belgium, 7Department of Gastroenterology, AZ Maria Middelares, Gent, Belgium, 8Department of Gastroenterology, UZ Antwerp, Antwerp, Belgium, 9Department of Gastroenterology, AZ Damian, Oostende, Belgium, 10Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium
Background
Loss of response (LOR) to infliximab (IFX) remains a challenge in routine management of IBD patients. We evaluated IFX high-resolution pharmacokinetics (PK) during induction with intermediate and peak PK levels.
Methods
This is a prospective, multicentre (
Results
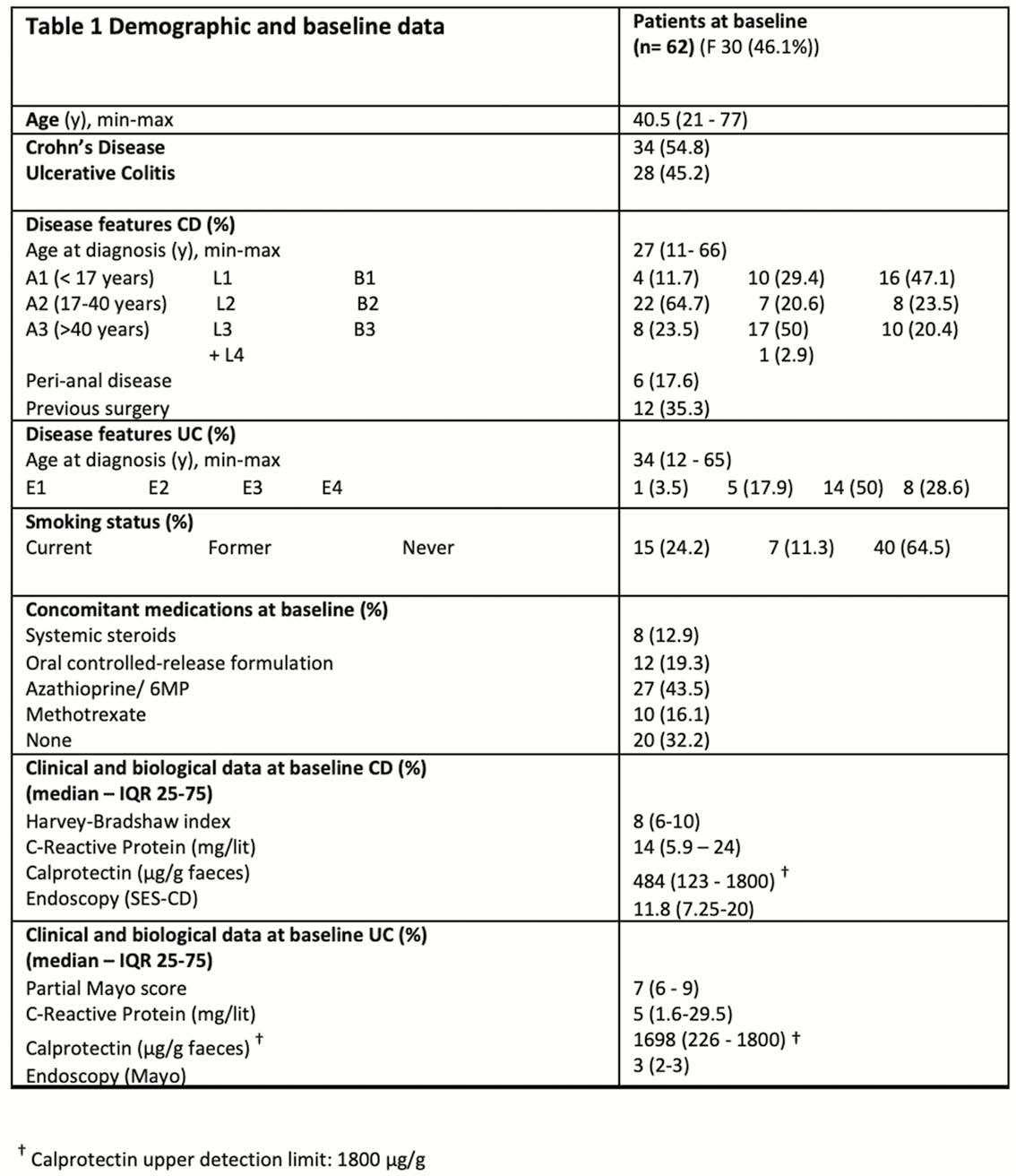
Demographic and baseline data of the study population are presented in Table 1.
Among the 62 patients enrolled, 33.9% of patients (
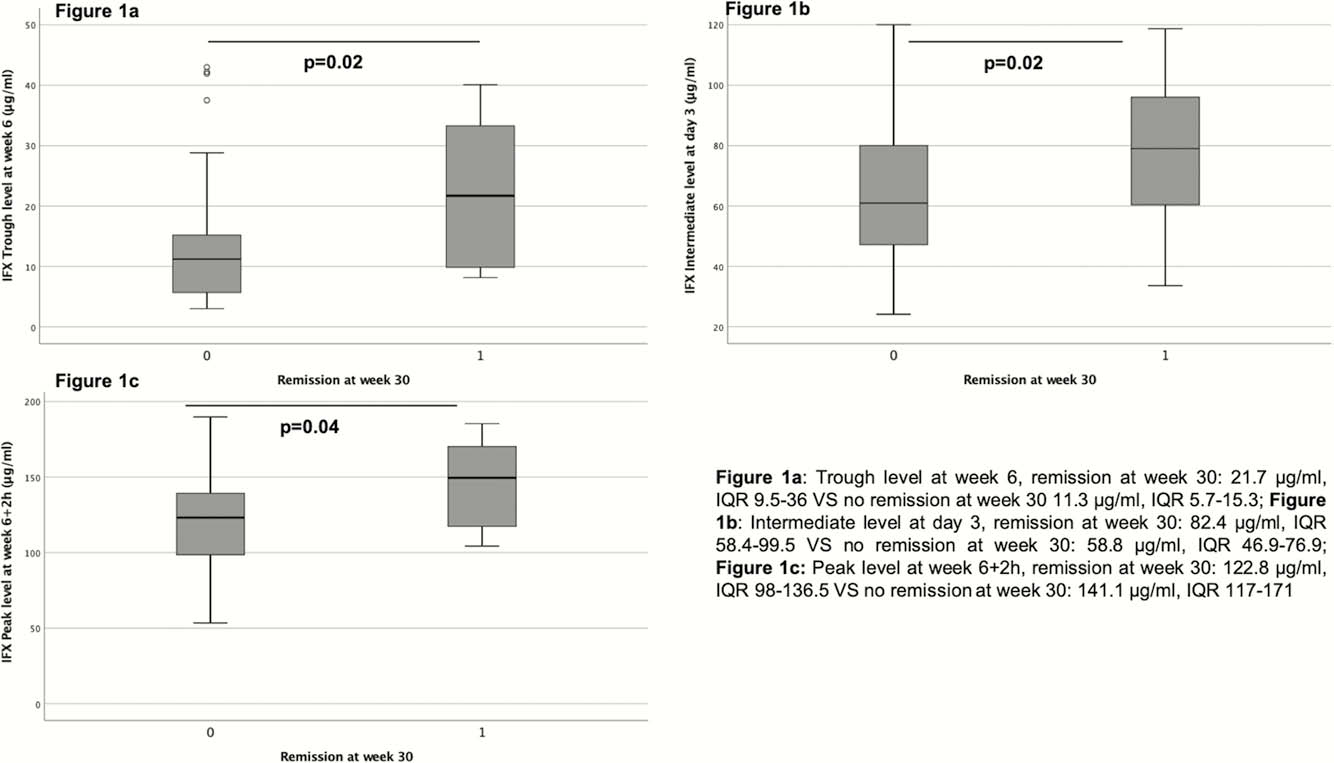
ATI were detected as soon as week 6. At week 2, infliximab levels were significantly lower among patients in which ATI developed at a later time point (
Conclusion
This multicentre prospective study demonstrates that intermediate levels as early as day 3 predict remission at week 30 in IBD patients. Low IFX levels during induction were correlated to future ATI development. PK modelling may allow to better select patients early on for sustained remission with infliximab.


