P374 Therapeutic drug monitoring can guide the intravenous-to-subcutaneous switch of infliximab and vedolizumab: a pharmacokinetic simulation study
Wang, Z.(1)*;Verstockt , B.(2,3);Sabino , J.(2,3);Ferrante , M.(2,3);Vermeire , S.(2,3);Dreesen, E.(1);
(1)KU Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium;(2)KU Leuven, Department of Chronic Diseases and Metabolism, Leuven, Belgium;(3)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;
Background
Subcutaneous (SC) formulations of infliximab (IFX) and vedolizumab (VDZ) were recently approved for maintenance treatment of patients with inflammatory bowel disease. However, further investigation is required to identify the patients most likely to benefit from intravenous (IV)-to-SC switch. We hypothesise that therapeutic drug monitoring (TDM) can guide the IV-to-SC switch.
Methods
Population pharmacokinetic (popPK) simulations were performed with previously published popPK models to explore the exposure profiles following various label and off-label IV-to-SC dosing scenarios (NONMEM 7.5).1-3 All virtual patients received IV IFX or VDZ at treatment weeks 0, 2, 6, 14, 22, and 30, and then switched to every two weeks (Q2W) or Q1W SC dosing.
Results
Following IV-to-SC switch according to label, the serum trough concentrations (Ctrough) will gradually increase over eight weeks (for IFX) and 16 weeks (for VDZ) before reaching the new steady state (SS) (Figure 1A-B). A linear relationship was observed between IV and SC Ctrough,ss for both drugs, allowing the prediction of SC Ctrough,ss hnbased on previous IV Ctrough,ss (Figure 1C-D).
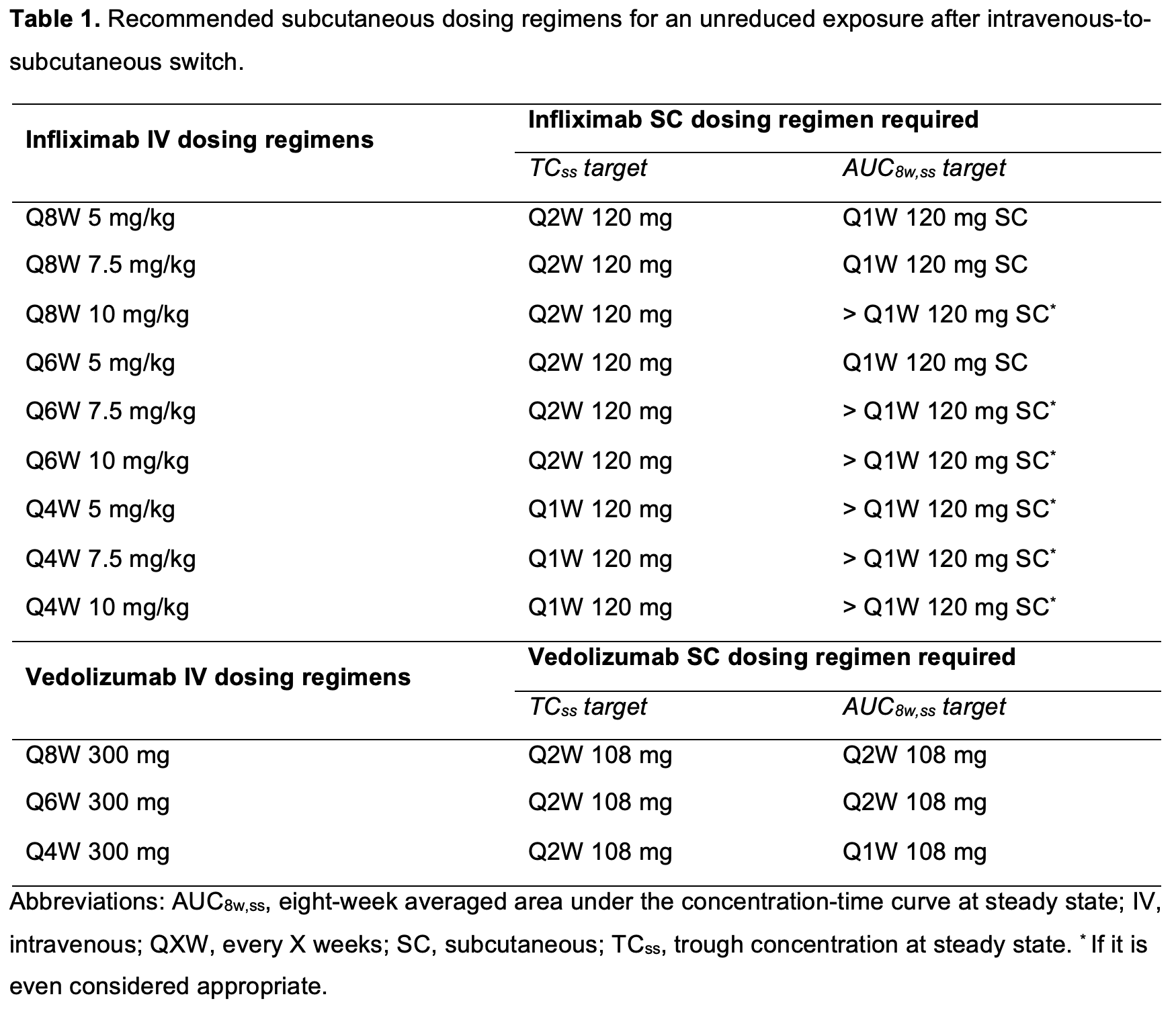
Administering the first SC dose four instead of eight weeks after the last IV dose will hit SS much faster (immediately for IFX and in two months for VDZ), thereby allowing earlier TDM and avoiding the risk of temporary underexposure (Figure 2). Patients on Q6W or Q8W IV infliximab can safely switch to Q2W SC infliximab without a meaningful decrease in Ctrough,ss, while patients on Q4W IV infliximab need Q1W SC infliximab (Figure 3A). Vedolizumab Ctrough,ss will always be maintained after switching to Q2W dosing (Figure 3B).
While the Ctrough,ss target is easily maintained, it is more challenging to guarantee a stable eight-week averaged area under the concentration-time curve at SS (AUC8w,ss) upon switch. Surprisingly, switching to Q2W SC infliximab resulted in a lower AUC8w,ss than all possible IV infliximab dosing regimens used in clinical practice (Figure 3C). Only patients on Q4W IV vedolizumab should switch to Q1W SC dosing (Figure 3D). Depending on the belief whether it is Ctrough,ss or AUC that drives therapeutic response, patients who were on escalated IV dosing regimens may need Q1W SC to guarantee similar exposure before versus after switch (Table 1).
Conclusion
We performed popPK simulations to inform clinicians how to integrate TDM of IFX and VDZ into their clinical practice as a tool to monitor patients and guide decision-making on the optimal dosing strategy.
REFERENCES:
1. EMA/CHMP/548703/2019. European Medicines Agency. 2019.
2. Rosario et al. Aliment Pharmacol Ther. 2015.
3. Rosario et al. J Crohns Colitis. 13, S357 (2019).