P384 Therapeutic drug monitoring: performance of the first lateral flow-based Point of Care test for the quantification of infliximab in a finger prick
Ametzazurra, A.(1);Pascual, J.(1);Del Rio, L.(1);Maguregui, A.(1);Nagore, D.(1);Ruiz-Argüello, M.B.(1);
(1)Progenika Biopharma - Grifols, Research & Development, Derio, Spain
Background
Promonitor Quick IFX is a lateral flow test (LFT) for the quantification of infliximab (IFX) in human whole blood (finger prick or venous) or serum in 20 minutes. This LFT is based on a sandwich immunoassay to quantify either the reference IFX or biosimilars. This abstract describes the studies performed to establish the analytical specifications of the product.
Methods
Clinical and Laboratory Standards Institute (CLSI) guidelines were followed for the evaluation of the analytical specifications of the LFT in whole blood and serum matrices: Linearity (EP-06-A), Detection capability (EP17-A2), Interfering substances (EP07, 3rd Edition), Intermediate precision (EP05-A3) and Bias evaluation study for Biosimilars (EP10-A3). Results were obtained in combination with the automated portable reader PQreader. A Datamatrix provided with each Promonitor Quick IFX kit contains the calibration information required for the PQreader to measure the Control and Test lines and report the IFX concentration.
Results
The linear assay range was determined to be 1-58 µg/mL in whole-blood and 0.6-67 µg/mL in serum according to the processes indicated in the Package Insert. The Limit of Blank is 0.8 μg/mL, the Limit of Detection and Lower Limit of Quantification (LLoQ) are 1.1 μg/mL, and the Upper Limit of Quantification (ULoQ) is 15.4 μg/mL.
There was no effect on assay performance when each of the following substances were added to samples with 0, 3, and 7 μg/mL of IFX: Haemoglobin (>1000 mg/dL), Bilirubin (>40 mg/dL), Triglycerides (>1500 mg/dL), HAMA (160 AU/mL), Rheumatoid factor (200 IU/mL), EDTA (5.4 mg/mL), Heparin (51 U/mL), Citrate (11.4%), Vedolizumab (60 μg/mL) and Adalimumab (20.25 μg/mL).
Repeatability and within-device precision results obtained for the positive samples are shown in the table below.
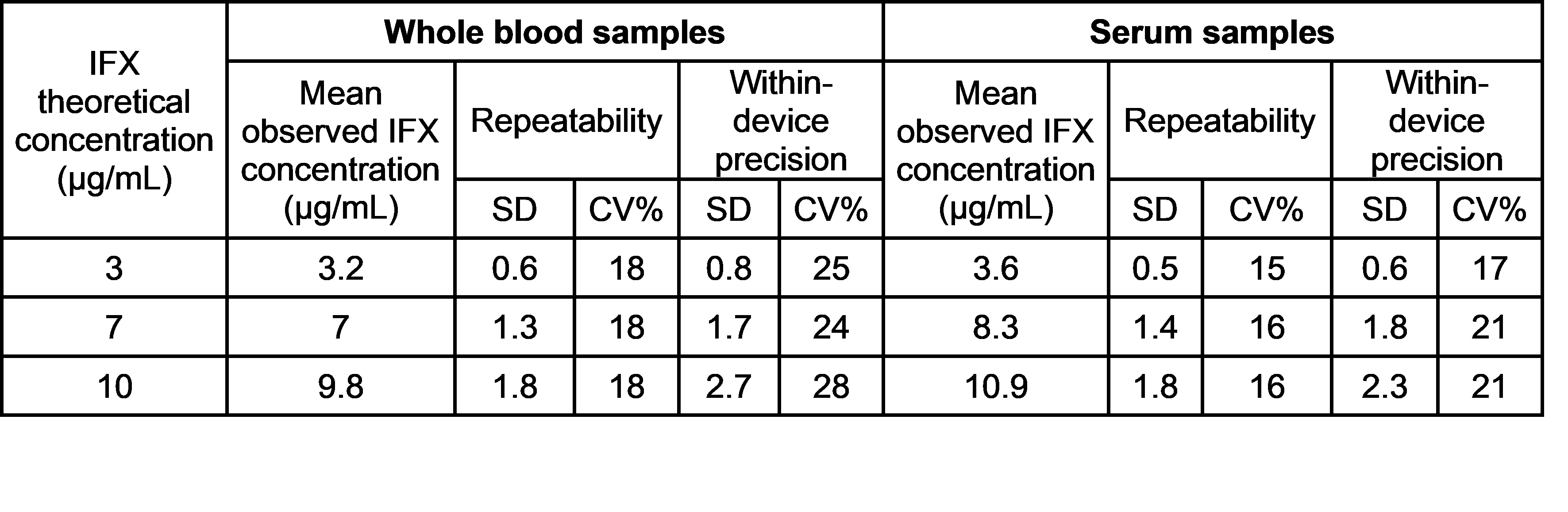
The negative samples showed a negative result in all the measurements.
A bias study showed that Promonitor Quick IFX can quantify CT-P13, SB2 and GP1111 biosimilars throughout the measurement range with a maximum bias of 14%.
Conclusion
Promonitor Quick IFX is the first LFT available for true Point of Care testing of patients treated with IFX with just a finger prick sample. It provides quick turnaround time to facilitate therapeutic drug monitoring and aid immediate decision making in the doctor office or hospitals with an excellent analytical performance.


