P393 Treatment patterns of patients with perianal fistulizing Crohn’s disease from a US administrative claims database
Cazzetta, S.(1);Chen, G.(2);Pedarla, V.(3);Null, K.(4);Rana Khan, Q.(1);Schwartz, D.(5);
(1)Takeda Pharmaceuticals USA- Inc., Medical Affairs, Lexington, United States;(2)At the time of the study Takeda Pharmaceuticals USA- Inc., Clinical Pharmacology, Lexington, United States;(3)Statinmed Research, Research, Plano, United States;(4)Takeda Pharmaceuticals USA- Inc., Outcomes Research & Data Science, Lexington, United States;(5)Vanderbilt University Medical Center, Inflammatory Bowel Disease Center, Nashville, United States
Background
Perianal fistula (PAF), a complication of Crohn’s disease (CD), is indicative of high disease severity and poor prognosis. We estimated the cumulative prevalence and treatment patterns of PAF CD in the USA.
Methods
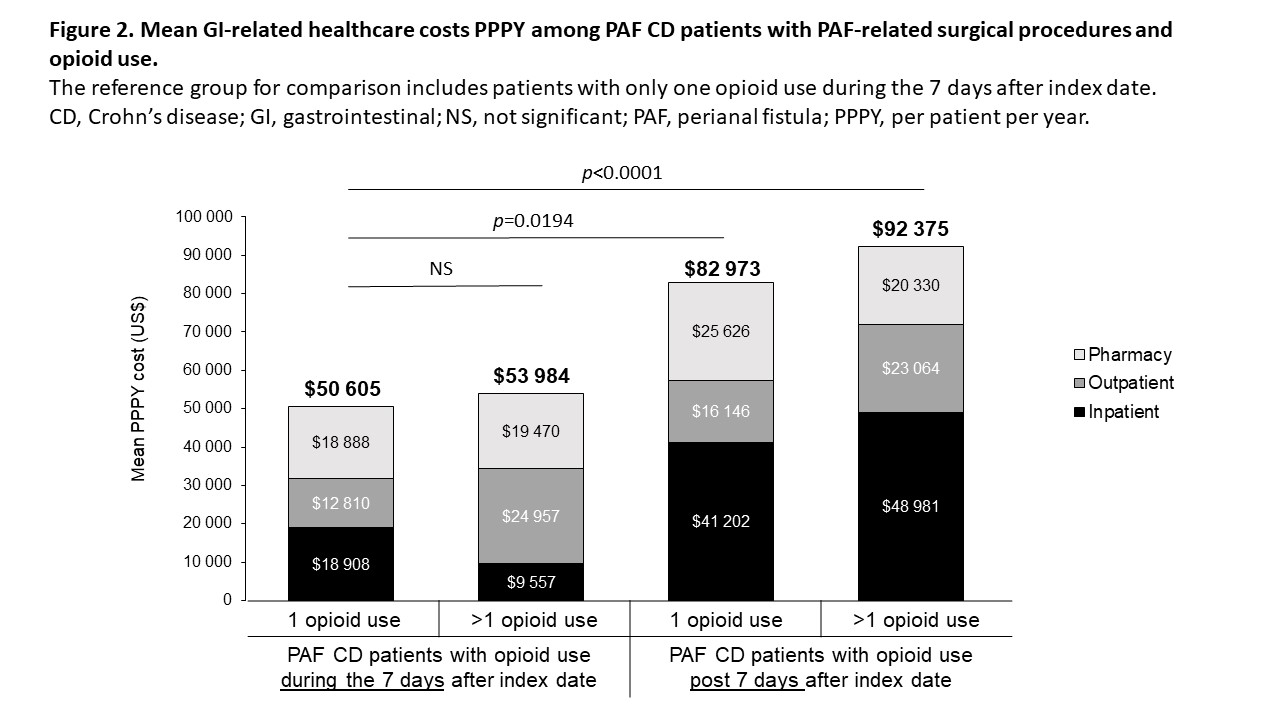
In this retrospective study of IBM® MarketScan® Commercial and Medicare databases (conducted 1 October 2015 to 30 September 2018), patients (pts) were 18 to 89 years of age with at least two diagnoses of CD at least 30 days apart, and had continuous health plan enrolment for at least 12 months pre- and post-index date (first PAF diagnosis or procedure [PAF pts]). Non-PAF CD pts were assigned the same index date as matched PAF pts based on birth year, sex, presence/lack of CD diagnosis before index date, CD disease location and follow-up duration. Descriptive analysis was used for all variables. Treatment patterns and costs related to opioid use were compared among PAF pts. We also assessed four PAF pt cohorts with PAF-related surgery treated with one (cohort 1) or more than one (cohort 2) opioid within 7 days of index date or one (cohort 3) or more than one (cohort 4) opioid more than 7 days after index date.
Results
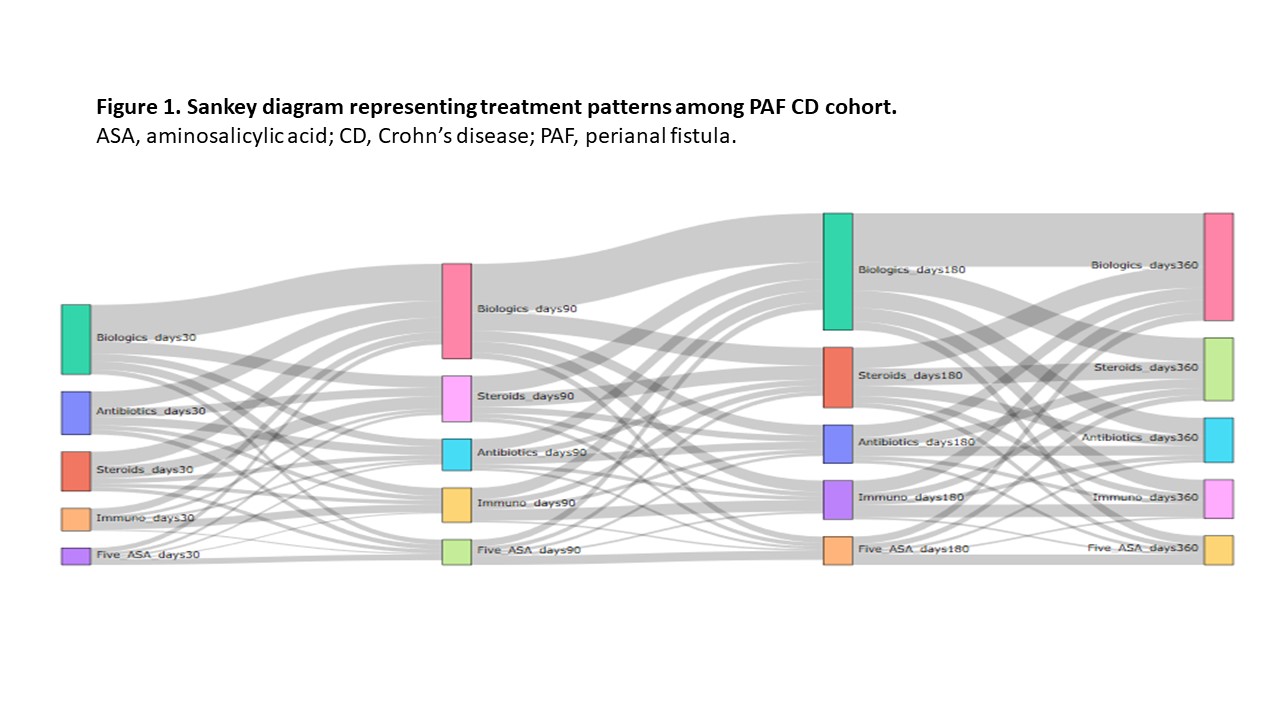
Cumulative prevalence of PAF CD (n = 81 862) was 7.7% (0.01% of the US population) over 3 years. The economic impact and treatment patterns were assessed in PAF (n = 1218; mean age 42 years; 52.4% men; 56.5% preferred provider organization [PPO] health plan) and matched non-PAF CD pts (n = 4095; mean age 43 years; 50.9% men; 57.6% PPO health plan). During follow-up, 65.8% of PAF and 42.3% of non-PAF pts were treated with at least one biologic agent. In the 30 days post-index, 31.9% of PAF pts were treated with biologics, with this percentage increasing over time; steroid use also remained high (Figure 1). Opioid treatment was associated with higher mean per patient per year (PPPY) total gastrointestinal (GI)-related costs for PAF pts (p < 0.0001). Mean PPPY total GI-related costs for pts with PAF-related surgery and opioid treatment were $50 605, $53 984, $82 973 and $92 375 for cohorts 1, 2, 3 and 4, respectively (Figure 2). Generalized linear model-adjusted mean PPPY PAF-related surgeries were 7.2 versus 0 for PAF pts and non-PAF pts (p < 0.0001), respectively. In the 30 days post-index date, 22.5% of PAF pts had minor surgeries and 20.0% had definitive surgeries.
Conclusion
Based on treatment guidelines as well as the study population’s use of inflammatory bowel disease medications and opioids, and higher rates of PAF-related surgeries, a need for better disease state management of patients with PAF CD is warranted.
Sponsor: Takeda Pharmaceuticals USA, Inc.