P396 Pharmacokinetics and circulating total lymphocyte count pharmacodynamic response from single and multiple oral doses of etrasimod in Japanese and Caucasian healthy male subjects
C.A. Lee1, L. Acevedo1, D.A. Oh1, P. Baweja1, K. Gilder1, D. Han2, S. Jhee2, K. Komori1, S. Mullin1, T. Nguyen-Cleary1, K. Schmelzer2, Y. Tang1, J. Zhang1, J. Grundy1
1Arena Pharmaceuticals Inc., n/a, San Diego, USA, 2PAREXEL International, n/a, Waltham, USA
Background
Etrasimod is a selective, sphingosine 1-phosphate receptor modulator in development for chronic immune-mediated inflammatory disorders. We evaluated etrasimod pharmacokinetics (PK) and its pharmacodynamic (PD) effect (lymphocyte count) in Japanese and Caucasian healthy male subjects.
Methods
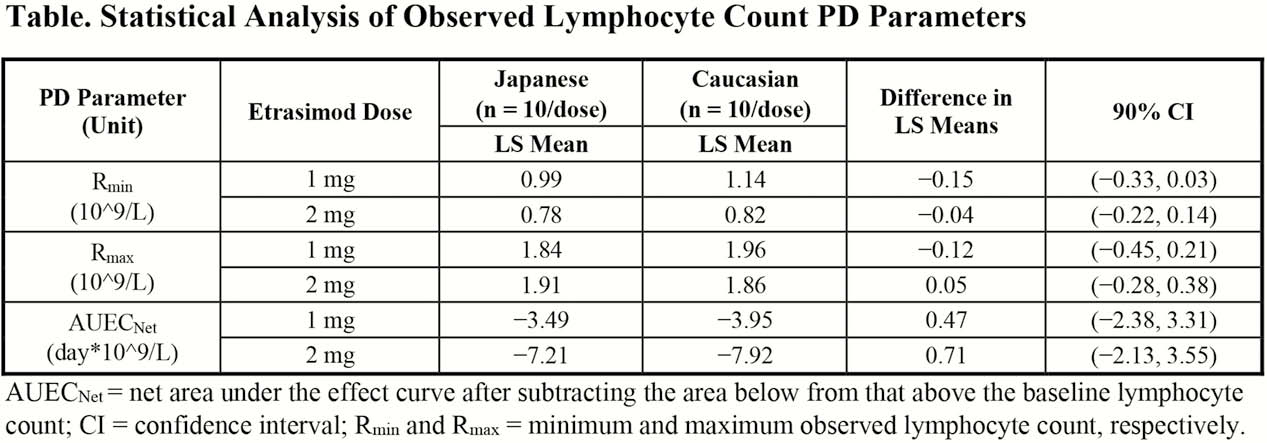
This phase 1 study comprised 12 men (10 etrasimod; 2 placebo) in each of 4 groups (Japanese, 1 and 2 mg; Caucasian, 1 and 2 mg). Etrasimod or matching placebo was administered once daily (QD) from Days 1 to 7, followed by a 7-day washout and a single dose on Day 15. Blood was intensively sampled on Days 1 and 7 for plasma PK and collected each morning on Days 1 to 15 to measure lymphocyte counts and calculate lymphocyte PD parameters, including Rmin, Rmax, and AUECNet.
Results
Etrasimod peak (Cmax) and total (AUC0-τ) plasma exposure values in Japanese and Caucasian subjects were dose-dependent and showed low-to-moderate inter-subject variability for each dose. Following single and multiple doses, geometric least squares (LS) mean etrasimod exposure values were slightly-to-moderately higher in Japanese subjects compared with Caucasian subjects; however, corresponding dose-body weight normalised etrasimod exposure values were similar indicating the exposure differences appear mainly attributable to bodyweight differences rather than ethnicity. Dose-dependent decreases in median lymphocyte counts were observed in both ethnicities from Days 2 to 8 and increased back to near baseline levels during the washout period. As expected, only LS mean Rmin and AUECNet values were dose-dependent in both ethnicities (table), with none of the evaluated lymphocyte PD parameters being statistically different between Japanese and Caucasian male subjects.
Conclusion
These results demonstrate the lack of clinically meaningful PK or PD (lymphocyte response) ethnic differences between Japanese and Caucasian healthy male subjects and support the potential inclusion of Japanese patients with moderately to severely active ulcerative colitis in global phase 3 clinical trials evaluating an etrasimod 2 mg QD dosing regimen.


