P399 Endoscopic and histologic outcome in tofacitinib treated refractory moderate-to-severe ulcerative colitis: A prospective real-life cohort
B. Verstockt1, A. Outtier1, J. Lefrère1, J. Sabino1, S. Vermeire1, G. De Hertogh2, M. Ferrante2
1IBD Leuven, 1Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium, 2Laboratory of Morphology and Molecular Pathology, University Hospitals Leuven, Leuven, Belgium
Background
The pan-Janus kinase inhibitor tofacitinib (TFC) has recently been approved for treatment moderate-to-severe ulcerative colitis (UC). Real-life data are limited, especially on endoscopic and histologic outcome. We report the efficacy and safety of TFC in refractory UC patients, and assessed potential clinical predictors of response.
Methods
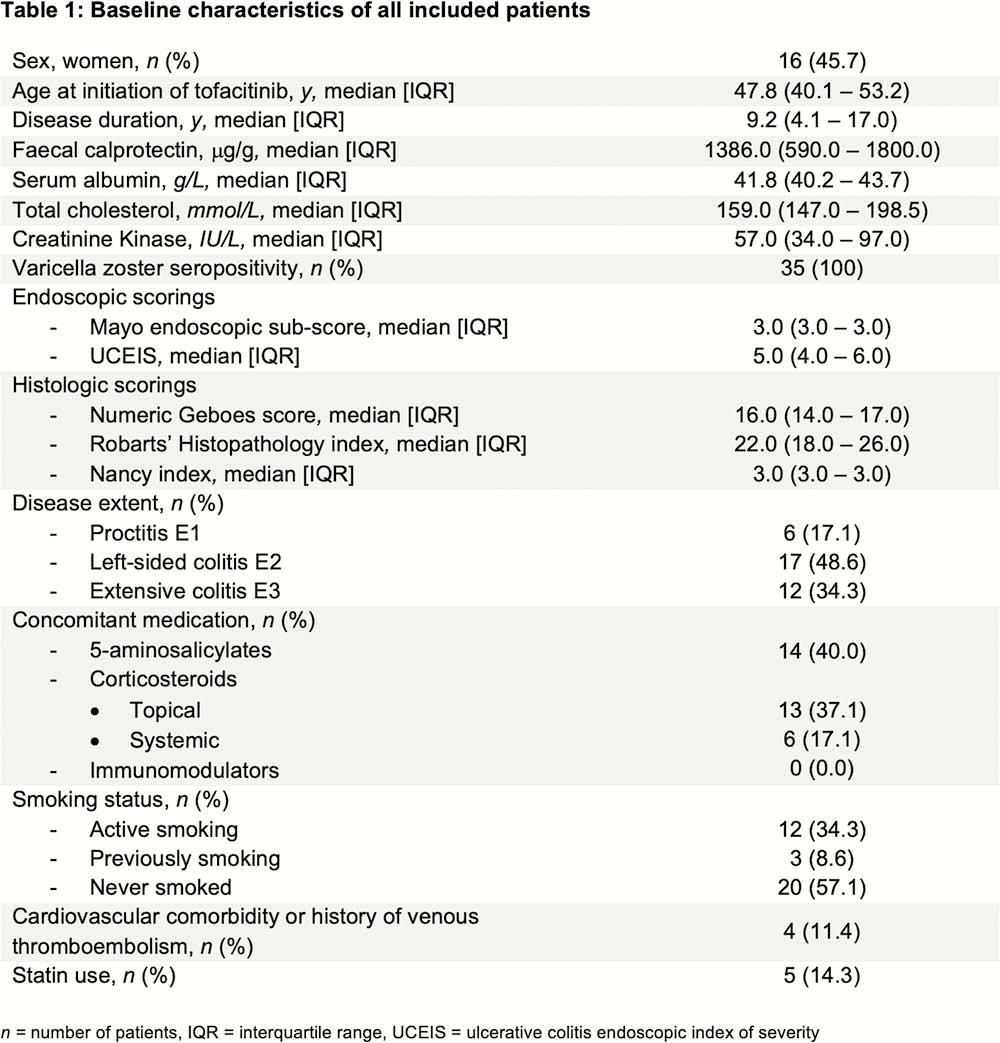
Thirty-five UC patients, all refractory to anti-TNF and vedolizumab, were prospectively included (Table 1). All received TFC 10mg BID till week 8, and were endoscopically assessed at baseline (Mayo endoscopic sub-score ≥2) and week 8. Biological response was defined as a 50% decrease in faecal calprotectin (fCal) or fCal <250 mg/g, and biological remission as a fCal <250 mg/g at week 8. The endoscopic response was defined as Mayo endoscopic sub-score of ≤1, endoscopic remission as a sub-score of 0. Histologic remission was defined as a numeric Geboes score ≤6 (similar to ≤2A.0). A non-response imputation and last observation carried forward analysis was applied.
Results
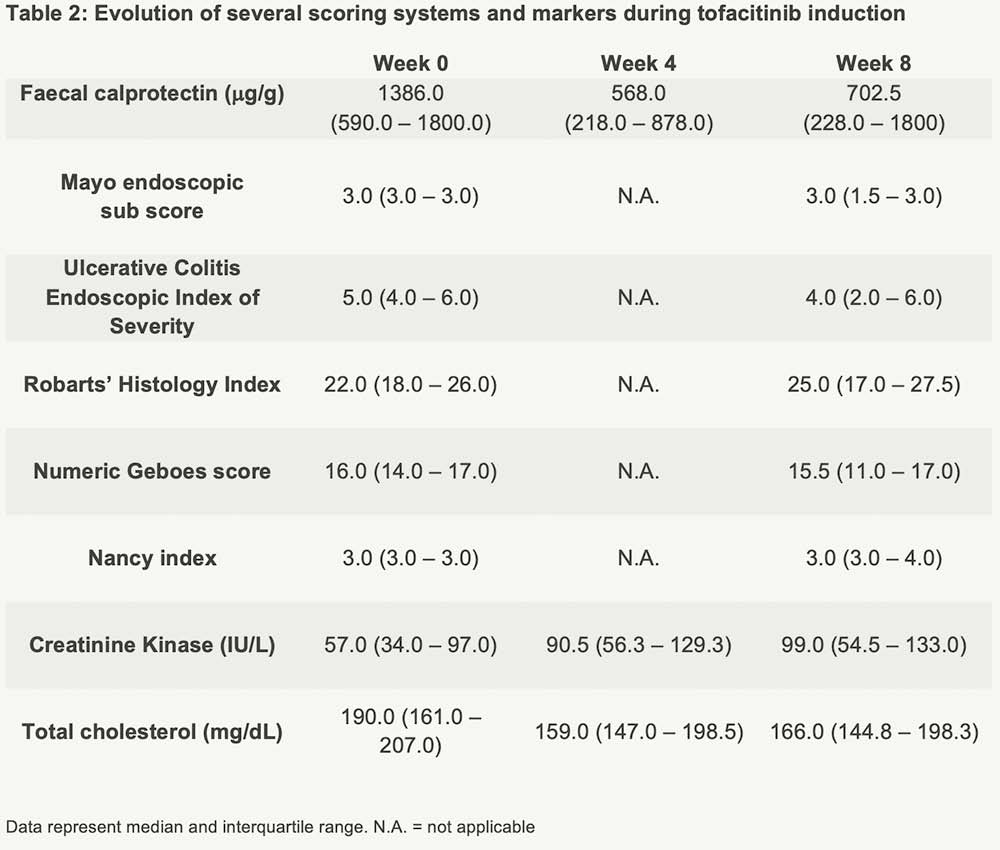
The Mayo endoscopic sub-score decreased significantly by week 8 (
Conclusion
TFC can induce biologic, endoscopic and histologic remission in refractory UC, though clinical and predictive molecular markers are required to identify the right patients. In patients with prior PNR to 2 anti-TNF agents, TFC does not seem an alternative treatment strategy.


