P416 Results of the first paediatric randomised controlled trial of faecal microbiota transplant for ulcerative colitis
N. Pai2, J. Popov3, L. Hill4, E. Hartung1, K. Grzywacz5, D. Godin5, L. Thabane6, C. Lee7, W. Khan7, M. Surette2,8,9, P. Moayyedi2,8
1McMaster Paediatric FMT Research Program, 1Division of Gastroenterology and Nutrition- McMaster Children’s Hospital, Paediatrics, Hamilton, Canada, 2Farncombe Family Digestive Health Research Institute, Department of Medicine- McMaster University, Hamilton, Canada, 3College of Medicine and Health, University College Cork, Cork, Ireland, 4Department of Exercise Science and Sports Medicine, University of Cape Town, Cape Town, South Africa, 5Division de Gastroentérologie- Hépatologie et Nutrition- Université de Montréal, Département de Pédiatrie, Montreal, Canada, 6Department of Health Research Methods- Evidence- and Impact, McMaster University, Hamilton, Canada, 7Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Canada, 8Department of Medicine, Division of Gastroenterology and Hepatology- McMaster University, Hamilton, Canada, 9Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, Canada
Background
The role of faecal microbiota transplant (FMT) for the treatment of ulcerative colitis (UC) has been reported across 4 randomised-controlled trials (RCT) in adults. Promising data have emerged from small, open-label paediatric case series and case reports but a proper blinded, placebo-controlled RCT has not been described in children. We report results from the first multicentre RCT of FMT in paediatric UC patients, conducted over 36 months in Ontario and Quebec, Canada.
Methods
We enrolled 25 children, ages 4–17 years old with active UC across two tertiary IBD clinics. Patients had active inflammation and remained on stable doses of medication at entry. Blinded participants received enemas containing healthy donor stool (active) or normal saline (placebo), 2×/week for 6 weeks. Faecal calprotectin (fCal), C-reactive protein (CRP), and paediatric ulcerative colitis activity index (PUCAI) scores were compared between groups during intervention, and at four follow-up time points over 30 weeks. Donor and recipient stools were measured for 16s rRNA and metagenomics analyses.
Results
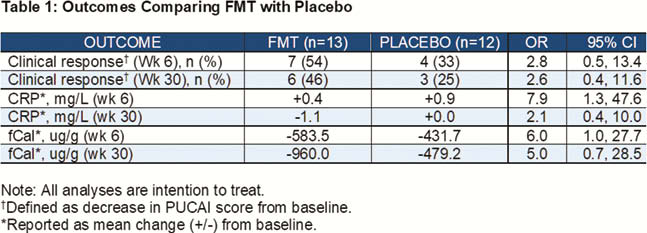
In intention-to-treat (ITT) analysis, FMT (
Conclusion
Serial FMT enemas containing healthy donor microbiota led to greater improvements in serum and stool inflammatory markers, and rates of clinical response, in paediatric patients with active UC compared with placebo. These improvements largely persisted beyond 6 months after final FMT treatment. This study offers the strongest preliminary evidence, from a blinded, placebo-controlled multicentre RCT for the role of FMT in the management of paediatric UC.


