P419 Clinical effectiveness and safety of first-line biologic vedolizumab as a monotherapy or combination therapy in ulcerative colitis and Crohn’s disease patients: results from the EVOLVE study
B. Bressler1, A. Yarur2, U. Kopylov3, M. Bassel4, N. Brett4, C. Colby5, C. Lopez6, S. Saha7, C. Kifnidi8, C. Agboton9, S. Adsul9, D. Demuth10, M. Luo11, S. Wang11, G.J. Mantzaris12
1Gastroenterology, St. Paul’s Hospital, Vancouver, Canada, 2Gastroenterology, Medical College of Wisconsin, Milwaukee, USA, 3Gastroenterology, Sheba Medical Center, Tel Aviv, Israel, 4Evidera, Real-World Evidence, Montreal, Canada, 5Evidera, Real-World Evidence, San Francisco, USA, 6Takeda Pharmaceuticals USA Inc., Medical Affairs, Deerfield, USA, 7Takeda Canada Inc., Medical Affairs, Oakville, Canada, 8Takeda Hellas S.A, Medical Affairs, Athens, Greece, 9Takeda Pharmaceuticals International AG, Global Medical Affairs, Zurich, Switzerland, 10Takeda Pharmaceuticals International, Evidence Generation and Publications, Singapore, Singapore, 11Takeda Pharmaceuticals International, Global Outcomes Research, Boston, USA, 12Evangelismos Hospital, Gastroenterology, Athens, Greece
Background
There is little long-term research (≥12 months) in ulcerative colitis (UC) and Crohn’s disease (CD) patients investigating the impact on clinical effectiveness of combined (combo) therapy of vedolizumab (VDZ) plus immunomodulators/immunosuppressants (IMMs) compared with VDZ monotherapy. Research suggests the use of concomitant aminosalicylates [5-ASAs] in UC may not bolster effectiveness. Finally, it is unclear if the safety profile differs between VDZ monotherapy and combo therapy. This study described clinical effectiveness and safety outcomes in patients with UC or CD treated with first-line biologic VDZ as monotherapy or combo therapy with IMMs or 5-ASAs (UC only).
Methods
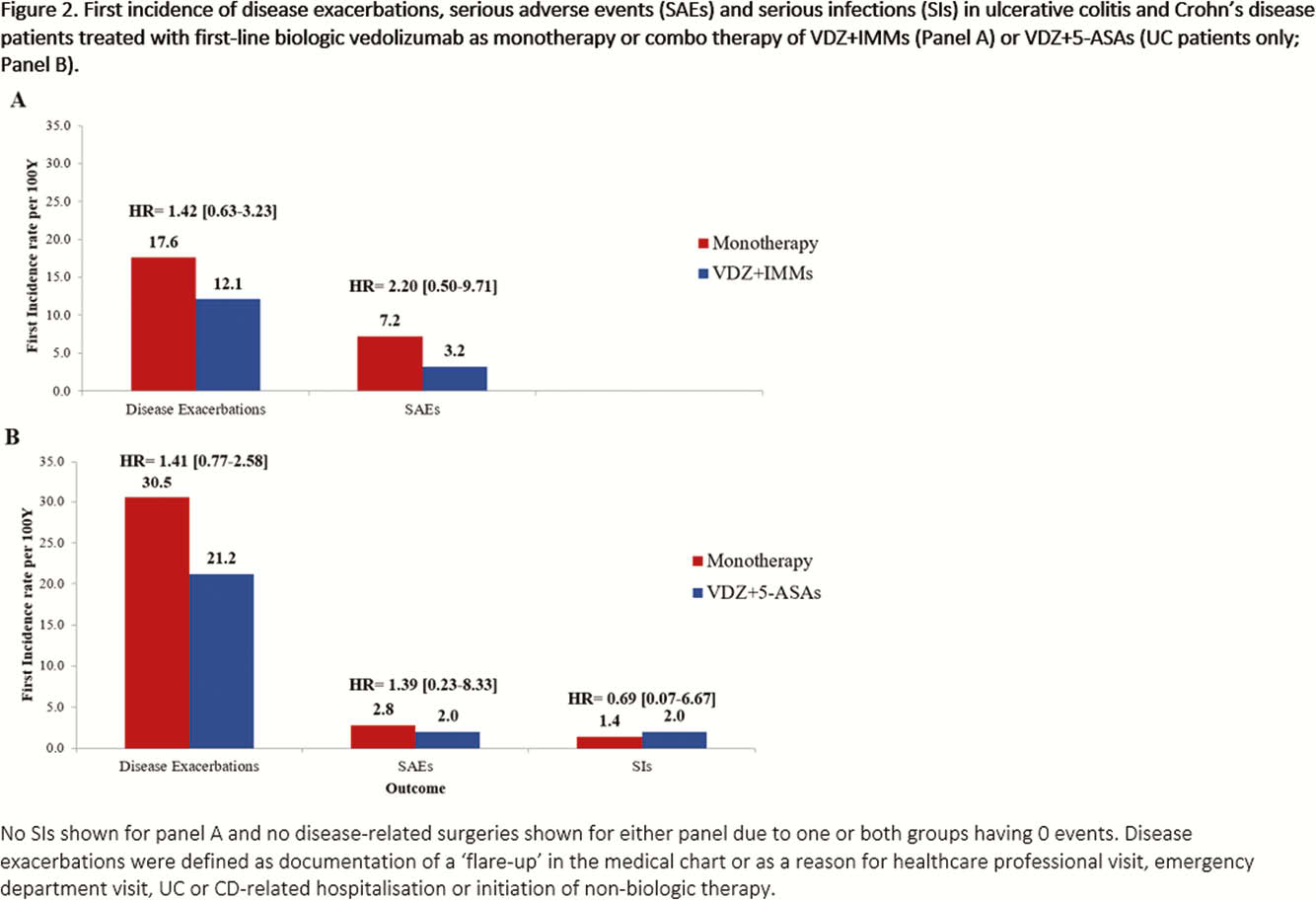
This was a real-world, multi-country (Canada, Greece and the USA), retrospective chart review study of biologic-naïve UC and CD patients (≥18 years old) treated with VDZ (initiated Tx May 2014–March 2018). Data were collected from Tx initiation to the earliest of death and chart abstraction date. Cumulative rates of clinical effectiveness outcomes over 24 months (Tx persistence, clinical response and clinical remission) were estimated using the Kaplan-Meier method with unadjusted comparisons conducted using the log-rank test. Clinical response and remission were assessed from standard disease measures reported in medical records. Analyses of unadjusted incidence rates (per 100 person-years [PYs]) of disease exacerbations, disease-related surgeries, serious adverse events (SAEs) and serious infections (SIs) were performed. For these analyses in monotherapy vs. VDZ+IMMs, UC and CD patients were combined due to restrictions of sample size and a number of events.
Results
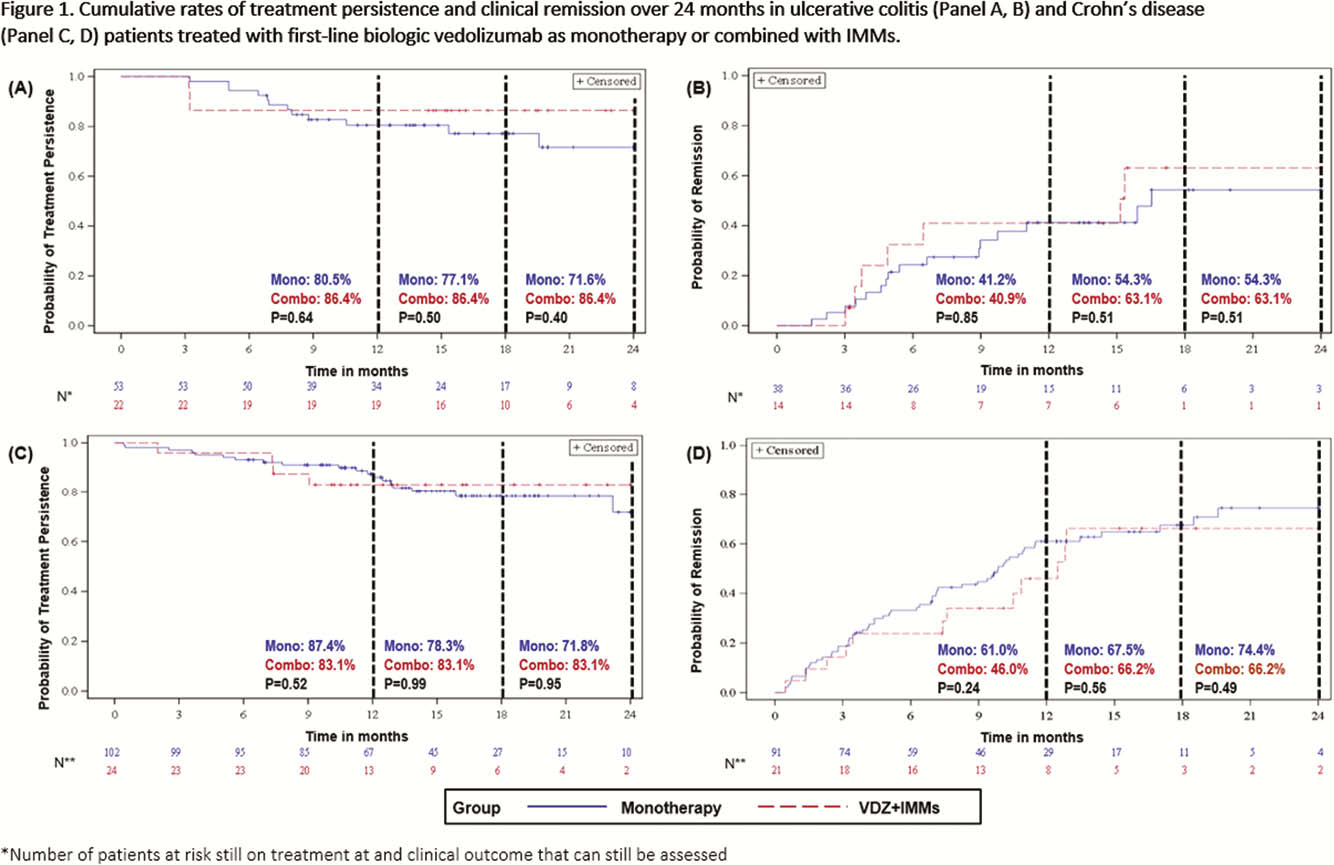
This analysis included 318 patients treated with VDZ (monotherapy: UC = 53, CD = 108; VDZ+IMMs: UC = 22, CD = 24; VDZ+5-ASAs: UC = 111). There were no observed differences in age, sex or disease duration between patients on monotherapy vs. VDZ+IMMs or vs. VDZ+5-ASAs. Data trends in effectiveness outcomes were similar in monotherapy vs. VDZ+IMMs over 24 months (Figure 1). Tx persistence (monotherapy: 71.6%; VDZ+5-ASAs: 82.7%;
Conclusion
Though sample sizes were small, the unadjusted trends in the results of this long-term real-world study suggest that biologic-naïve UC or CD patients treated with VDZ alone may have similar clinical effectiveness outcomes to patients receiving VDZ+IMMs. Trends in data also suggest that in patients with UC, VDZ+5-ASAs may not be more effective than VDZ alone.


