P422 Tofacitinib induces clinical and endoscopic remission in biologic refractory ulcerative colitis patients: a real-world Belgian cohort study
A. Cremer1, T. Lobaton2, S. Vieujan3, P. Bossuyt4, J.F. Rahier5, F. Baert6, O. Dewit7, M. Somers8, E. Macken8, A. Vijverman9, P. Van Hootegem10, F. Mana11, B. Willandt12, P. Caenepeel13, E. Humblet13, F. D’Heygere14, A. Verreth15, A. El Nawar16, J.L. Coenegrachts17, S. Dewit18, S. De Coninck19, N. Schoofs20, S. Delen21, J. Dutre22, C. Thienpont23, S. Vanden Branden24, D. Staessen25, D. Franchimont1, Belgian Inflammatory Bowel Disease Research and Development (BIRD) Group
1Department of Gastroenterology, Erasme University Hospital- ULB, Brussels, Belgium, 2Department of Gastroenterology, University Hospital Ghent, Ghent, Belgium, 3Department of Gastroenterology, CHU Sart Tilman, Liège, Belgium, 4Department of Gastroenterology, Imelda General Hospital, Bonheiden, Belgium, 5Department of Gastroenterology, CHU UCL Namur, Yvoir, Belgium, 6Department of Gastroenterology, AZ Delta, Roeselare, Belgium, 7Department of Gastroenterology, Clin Univ Saint Luc, Brussels, Belgium, 8Department of Gastroenterology, University Hospital Antwerp, Edegem, Belgium, 9Department of Gastroenterology, CHR de la Citadelle, Liège, Belgium, 10Department of Gastroenterology, AZ Sint-Lucas, Brugge, Belgium, 11Department of Gastroenterology, Clinique Saint-Jean, Brussels, Belgium, 12Department of Gastroenterology, AZ Sint-Jan, Brugge, Belgium, 13Department of Gastroenterology, Ziekenhuis Oost-Limburg Sint-Jan, Genk, Belgium, 14Department of Gastroenterology, AZ Groeninge, Kortrijk, Belgium, 15Department of Gastroenterology, AZ Sint-Jozef, Malle, Belgium, 16Department of Gastroenterology, CH Mouscron, Mouscron, Belgium, 17Department of Gastroenterology, Jessa ZH, Hasselt, Belgium, 18Department of Gastroenterology, Maria ZH Noord-Limburg, Overpelt, Belgium, 19Department of Gastroenterology, Sint-Andries ZH, Tielt, Belgium, 20Department of Gastroenterology, Sint-Trudo ZH, Sint-Truiden, Belgium, 21Department of Gastroenterology-, ZH Maas en Kempen, Maaseik, Belgium, 22Department of Gastroenterology, ZNA Jan Palfijn, Merksem, Belgium, 23Department of Gastroenterology, ZNA Antwerpen, Antwerpen, Belgium, 24Department of Gastroenterology, Onze-Lieve-Vrouwziekenhuis, Aalst, Belgium, 25Department of Gastroenterology, GZA Sint-Vincentius ZH, Antwerpen, Belgium
Background
Tofacitinib, an oral small molecule Janus kinase inhibitor, has been approved in 2018 for the treatment of moderate to severe ulcerative colitis (UC) in Europe. We report on real-world short-term efficacy and safety data from a multicenter Belgium refractory cohort of UC patients with prior exposure to both anti-TNFα and vedolizumab.
Methods
This is an observational, national, retrospective multicentre study including all UC active patients started on tofacitinib (10 mg BID) from 25 centres in Belgium between November 2018 and August 2019. Prospectively collected data were retrospectively analysed according to intention to treat. Primary endpoints were clinical and endoscopic response and remission rates at weeks 8 and 16. Clinical response and remission were defined as a reduction in the Modified Clinical Mayo score (rectal bleeding, stool frequency) of ≥2 and ≤1, respectively. Endoscopic response and remission were defined as a reduction in Endoscopic Mayo score of ≥1 and ≤1, respectively. Complete endoscopic remission was defined as an Endoscopic Mayo score of 0. Descriptive statistics and Wilcoxon signed-rank test were calculated using Medcal 19.1.
Results
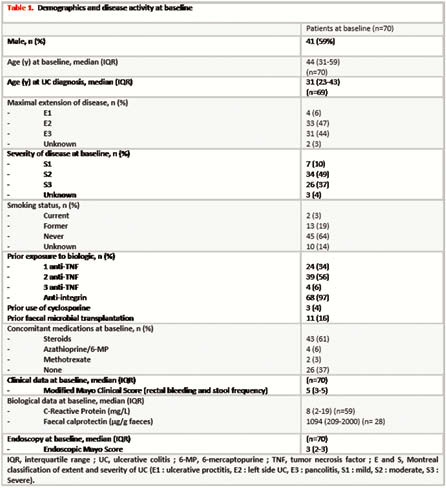
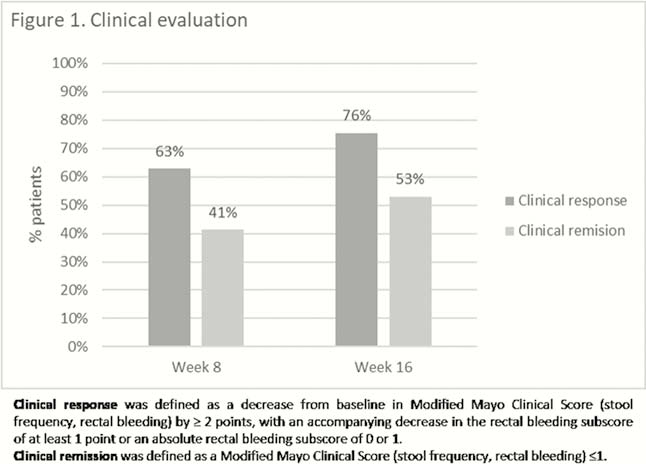
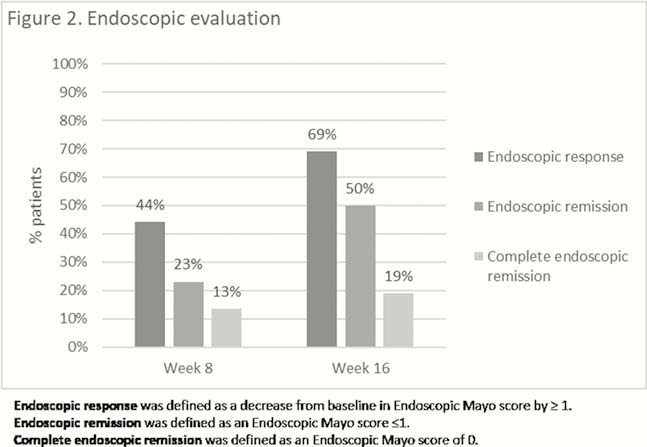
Demographic and baseline data of the 70 included patients are presented in Table 1. Of note is that nearly all patients were refractory to at least one anti-TNF and vedolizumab. Median follow-up was 16 weeks (IQR 13–26). Fifty-four per cent (38/70) of patients required prolonged induction at 10mg BID. Clinical evaluation was available in all patients at week 8 and 49 patients at week 16, while endoscopic data were available in 52 patients and 42 at weeks 8 and 16, respectively. Clinical response and remission, and endoscopic response and remission at weeks 8 and 16 are presented in Figures 1 and 2. Fifty per cent (21/42) of the patients under steroids at baseline could have stopped steroids at 16 weeks. Median baseline Modified Mayo score (rectal bleeding, stool frequency and endoscopy) decreased from 7 (IQR 5–8) to 4 (IQR 2–7) after 8 weeks (
Conclusion
Tofacitinib very effectively induced short-term clinical and endoscopic response and remission even in a refractory cohort of patients with UC in a real-world clinical setting. During this short-term follow-up, tofacitinib was well tolerated with respect to adverse events.


