P429 Clinical utility of thiopurine metabolite monitoring in inflammatory bowel disease and its impact on healthcare utilisation in Singapore
J.Q. Yeo1, H.L. Wee1, H.H. Cheen2, A. WONG2, T.G. Lim2, W.F. Leong3, B. Chowbay3, E. Salazar4, H.H. Shim4, W.P.W. Chan4, S.C. Kong5, W.C. Ong2
1Department of Pharmacy- Faculty of Science, National University of Singapore, Singapore, Singapore, 2Department of Pharmacy, Singapore General Hospital, Singapore, Singapore, 3Clinical Pharmacology, Singhealth, Singapore, Singapore, 4Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore, Singapore, 5Department of Gastroenterology and Hepatology, Sengkang General Hospital, Singapore, Singapore
Background
Thiopurines are recommended for maintenance of steroid-free remission in inflammatory bowel disease (IBD). Thiopurine metabolite monitoring (MM) is increasingly used in the Western population. However, it remains a new strategy in Singapore with limited information on its therapeutic and economic benefits. Hence, this study aims to investigate the clinical utility of MM and its impact on healthcare resource utilisation in Singaporean IBD patients.
Methods
A retrospective observational study was conducted at the Singapore General Hospital outpatient IBD Centre. Patients with IBD, baseline MM during 2014–2017 and weight-based thiopurine doses for ≥4 weeks were followed up for 1 year. Actions taken to optimise therapy, metabolite levels before and after the first action were documented. Outcomes assessed included steroid-free remission (SFR), clinical remission (defined as SFR, CRP <9.1mg/l and stool calprotectin <250ug/g), no escalation to anti-tumour necrosis factor or surgery, clinical healthcare resource utilisation and direct healthcare costs.
Results
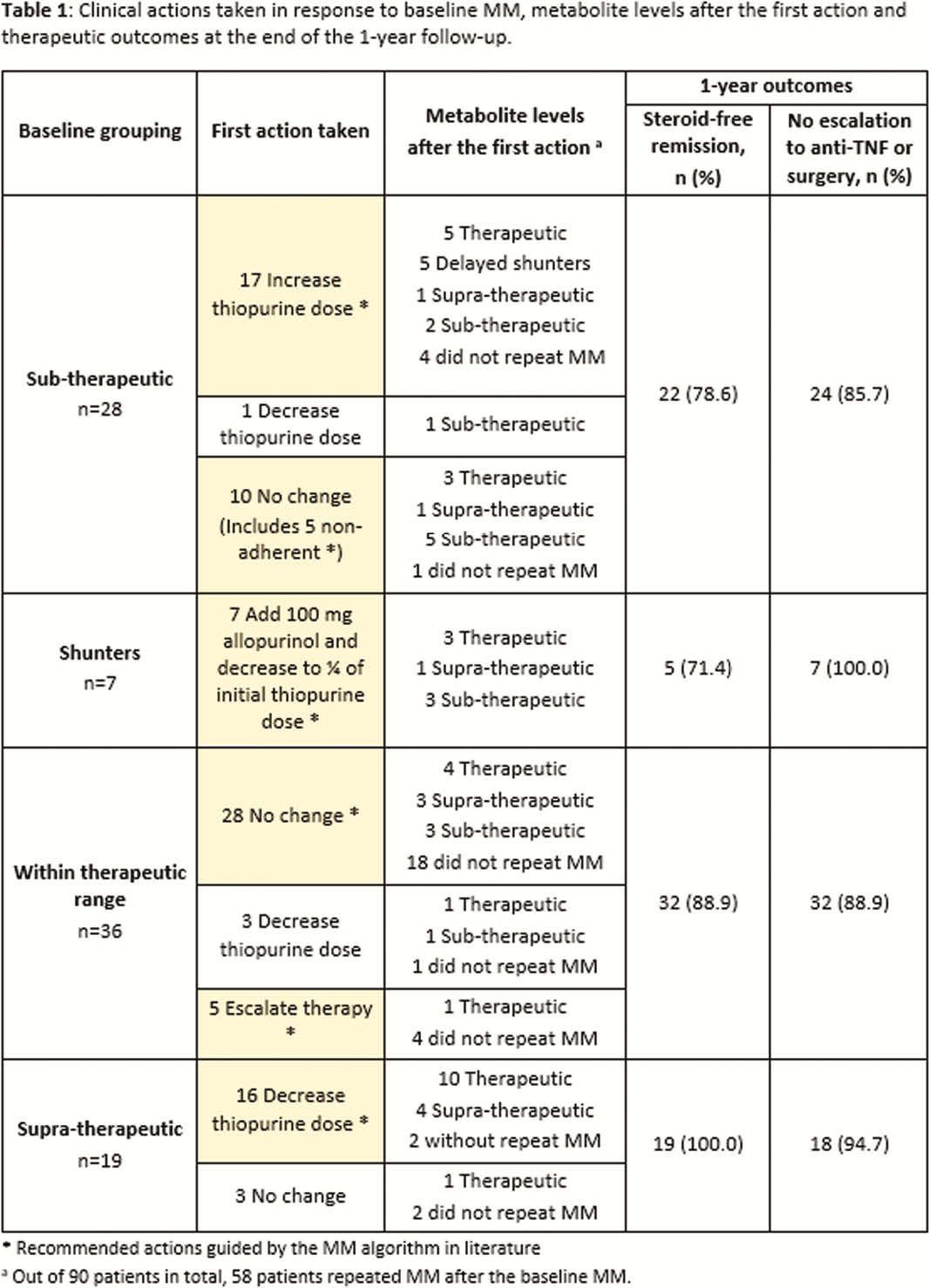
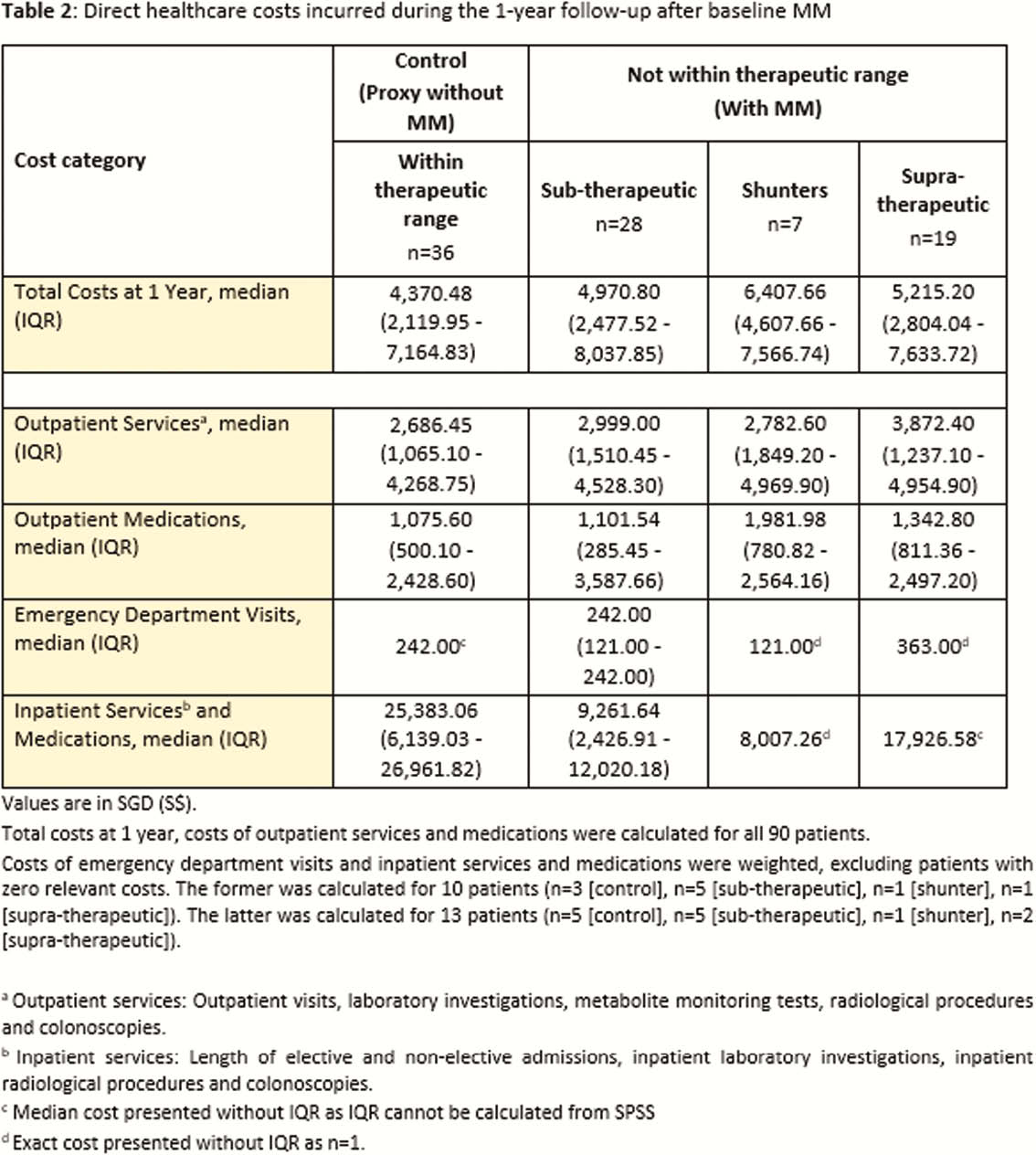
Ninety IBD patients (50 CD, 40 UC) were included. Among them, 40% had baseline metabolite levels within therapeutic range, 31.1% sub-therapeutic, 21.1% supra-therapeutic and 7.8% shunters. Repeated MM with subsequent dose optimisation helped 67.2% of patients achieve therapeutic levels after 1 year. In particular, dose optimisation and reinforcement of adherence in the sub-therapeutic group recaptured clinical remission in almost 50% of the patients. Overall, 86.7% of patients achieved steroid-free remission and 90% had no therapy escalation or surgery (Table 1). Despite greater outpatient visits and laboratory investigations with MM, the median total healthcare costs at 1 year only increased marginally (S$6,407.66 [shunters] vs. S$5,215.20 [supra-therapeutic] vs. S$4,970.80 [sub-therapeutic] vs. S$4,370.48 [control (within therapeutic range)],
Conclusion
MM guided timely dose optimisation or therapy escalation for non-responders, identification of non-adherence and reversal of shunting. Therefore, it is a useful clinical tool to optimise thiopurines without significantly increasing healthcare resource costs.


