P441 Clinical efficacy, drug sustainability and results from therapeutic drug monitoring in Crohn’s disease patients treated with ustekinumab – 1-year follow-up of a prospective, nationwide, multicenter cohort from Hungary
Gonczi, L.(1);Szanto, K.(2);Farkas, K.(2);Molnar, T.(2);Szamosi, T.(3);Schafer , E.(3);Golovics, P.(3);Lovasz, B.(1);Patai, A.(4);Vincze, A.(5);Sarlos, P.(5);Farkas, A.(6);Dubrovcsik, Z.(6);Tóth G., T.(7);Lakatos, P.L.(8);Miheller, P.(9);Ilias, A.(1);
(1)Semmelweis University, 1st Department of Medicine, Budapest, Hungary;(2)University of Szeged, 1st Department of Internal Medicine, Szeged, Hungary;(3)Military Hospital—State Health Centre, Department of Gastroenterology, Budapest, Hungary;(4)Markusovszky Hospital, Department of Medicine and Gastroenterology, Szombathely, Hungary;(5)University of Pecs, First Department of Medicine, Pecs, Hungary;(6)Bács-Kiskun County Hospital, Department of Gastroenterology, Kecskemét, Hungary;(7)St. Janos Hospital, Department of Gastroenterology, Budapest, Hungary;(8)McGill University Health Centre, Division of Gastroenterology, Montreal, Canada;(9)Semmelweis University, 1st Department of Surgery, Budapest, Hungary
Background
Although efficacy and safety of ustekinumab (UST) in the treatment of inflammatory bowel disease have been demonstrated through randomized trials, data from real-life prospective cohorts are still of great interest. Our aim was to evaluate the clinical efficacy, drug sustainability, frequency of dose intensification, and results from therapeutic drug monitoring in UST treated Crohn’s disease (CD) patients using a prospective, nationwide, multicenter cohort from Hungary.
Methods
Patients were consecutively enrolled in this cohort between 2019 January and 2020 May from 5 academic centers and 5 county hospitals. Data from patient demographics, disease phenotype, treatment history (surgical history, prior and present medical therapies), clinical disease activity (using the Crohn’s Disease Activity Index (CDAI), Harvey Bradshaw Index (HBI)), biomarkers (C-reactive protein – CRP), and therapeutic drug monitoring were captured. Evaluations were performed at week8 (post-induction), w16-20, w32-36, and w52-56 follow-up visits.
Results
N=142 CD patients were included with a median follow-up time of 60 weeks (IQR:47.5-79.5w) [57.4% female; age 38.4±13.0 years]. Based on the Montreal classification, complicated disease behavior (B2orB3) was 48.2%, whereas ileocolonic disease location(L3) 55.7%. Perianal manifestation was present in 46.8% of the patients. Previous anti-TNF exposition was 97.2%, while previous vedolizumab failure was 25.5%. 66.2%/ 66.9% of the patients had moderate-to-sever clinical disease activity at baseline (CDAI>220/HBI>7). Clinical response and remission rates were 78.1% and 57.7% using CDAI, and 82.5% and 51.8% based on HBI scores after induction treatment (w8). One year clinical remission rates were 58% / 57.3% (CDAI/HBI) Composite clinical and biomarker remission (CDAI<150 and CRP<10mg/L) rates were 35.4%; 33.3%; 38.6% and 36.6% at w8/w16-20/w32-36 and w52-56. Parallel corticosteroid use was 34%/26.3%/16.5%/21%/16.9% at baseline and w8/w16-20/w32-36/w52-56. Drug sustainability was high with 81.9% (SD: 3.4) of patients remaining on treatment at one year.(Figure1) Probability of dose intensification was high and introduced early in the treatment, 42.2% (SD: 4.2) at ~w32 and 51.9% (SD: 4.4%) at 1y.(Figure2) Patients with complex disease phenotype (B2/B3) had higher probability for dose intensification (log-rank: p=0.042). Mean serum trough levels of UST were 4,28±3,35/ 1,35±1,42/ 0,82±0,65 and 1,13±0,74µg/mL measured at w8/w16-20/w32-36 and w52-56. ADAs were exceeding 1AU/mL in only 2 patients.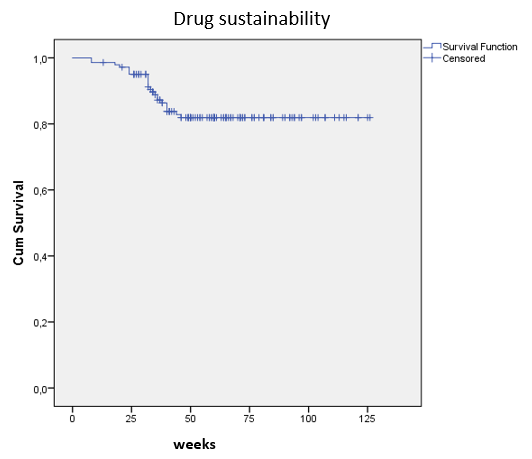

Conclusion
Ustekinumab showed good drug sustainability and clinical efficacy in a population with severe disease phenotype and high rates of previous anti-TNF failure, however frequent and early dose intensification was required.


