P448 Dose adjustment in patients with moderate-to-severe ulcerative colitis: results from the UNIFI maintenance study long-term extension
S. Danese1, R. Panaccione2, L. Peyrin-Biroulet3, C. Marano4, C.D. O’Brien4, H. Zhang5, Y. Zhou5, J. Johanns5, E. Scherl6, R.W.L. Leong7, D.S. Rowbotham8, R.P. Arasaradnam9, B.E. Sands10
1UNIFI Investigators, 1Humanitas Research Hospital, Inflammatory Bowel Diseases Center, Milan, Italy, 2Department of Medicine, University of Calgary, Calgary, Canada, 3Gastroenterology, Nancy University Hospital, Nancy, France, 4Janssen Research and Development- LLC, Immunology, Spring House, USA, 5Janssen Research and Development- LLC, Clinical Biostats, Spring House, USA, 6Weill Cornell Medicine, Jill Roberts Center for Inflammatory Bowel Disease, New York, USA, 7Gastroenterology, Concord and Macquarie University Hospitals, Sydney, Australia, 8Gastroenterology and Hepatology, Auckland City Hospital, Auckland, New Zealand, 9Gastroenterology, University Hospital Coventry and Warwickshire NHS Trust, Coventry, UK, 10Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, USA
Background
The UNIFI randomised-withdrawal maintenance study evaluated the safety and efficacy of subcutaneous (SC) ustekinumab (UST) in patients (patients) with moderately to severely active ulcerative colitis (UC) who had responded to intravenous (IV) UST during induction. We evaluated the efficacy of UST dose adjustment during the long-term extension (LTE).
Methods
At Week (Week) 0 of the 44 weeks maintenance study, 523 patients who had responded to IV UST induction were randomly assigned in a 1:1:1 ratio to placebo (PBO) SC, UST SC 90 mg q12w, or 90 mg q8w. Patients who completed the maintenance study were eligible to enter the LTE if the investigator thought they would benefit from continued treatment. PBO patients were discontinued from the LTE after the maintenance study was unblinded and the analysis was complete. Based on investigator’s clinical judgement of UC disease activity, patients in the LTE were eligible to receive dose adjustment starting at Week 56: PBO to q8w, q12w to q8w, and q8w to q8w (sham adjustment). PBO patients were only eligible for dose adjustment before unblinding. Patients were assessed for symptomatic remission (SR), partial Mayo scores, and inflammatory markers ≥16 weeks after dose adjustment.
Results
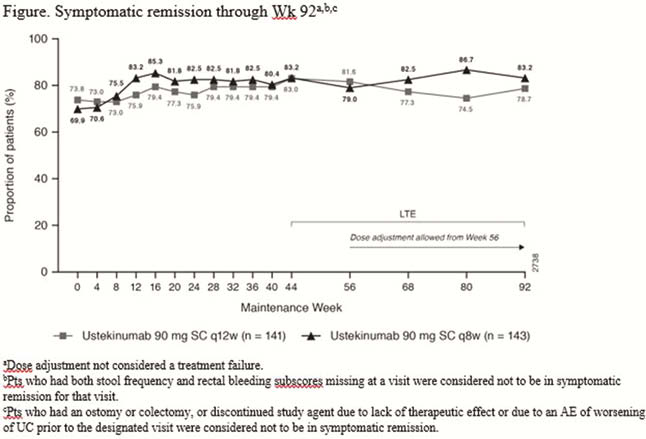
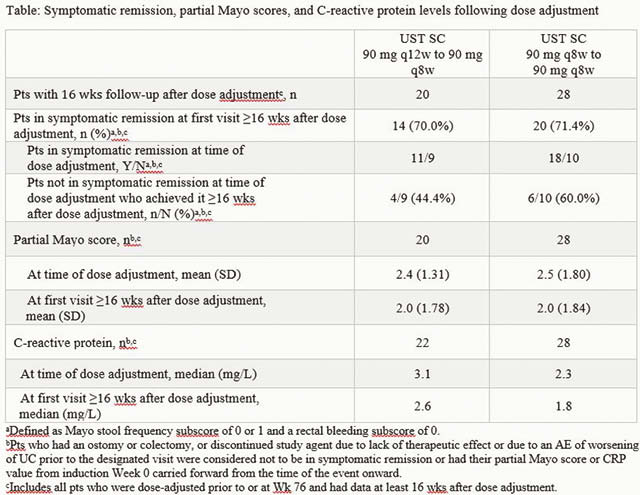
Symptomatic remission was maintained through Week 92 among patients treated with UST regardless of dose adjustment in the LTE (Figure). Overall, 40 (28.4%) and 37 (25.9%) patients in the q12w and q8w groups, respectively, underwent dose adjustment (or sham dose adjustment) prior to Week 92 of the LTE. Among patients who received dose adjustment at Week 76 or before and had data ≥16 weeks after dose adjustment, symptomatic remission was observed in 70.0% in the q12w-to-q8w group and 71.4% in the q8w-to-q8w group, the majority of whom were in symptomatic remission at the time of the dose adjustment (Table). The safety profile of UST in patients who received dose adjustment was generally consistent with the overall safety profile of UST.
Conclusion
Patients may benefit from UST dose adjustment to q8w. However, the majority of UST-treated patients were in SR at the time of dose adjustment in this study. No new safety signals were observed among patients who dose adjusted.


