P451 Evaluating drug sustainability, efficacy and safety of biologic agents in elderly patients with Inflammatory Bowel Disease: a single center retrospective study
Golovics, P.(1);LeBlanc, J.F.(2);Drügg Hahn, G.(1);Wetwittayakhlang, P.(1);Qatomah, A.(1);Wang, A.(1);Boodaghians, L.(1);Liu, J.(3); Al Ali, M.(1);Wild, G.E.(1);Afif, W.(1);Bitton, A.(1);Lakatos, P.L.(1);Bessissow, T.(1);
(1)McGill University Health Centre, Division of Gastroenterology, Montreal, Canada;(2)Sacre-Coeur Hospital, Division of Gastroenterology, Montreal, Canada;(3)University of Montreal, Department of Gastroenterology, Montreal, Canada;
Background
Despite the advancement in pharmacological management of Inflammatory Bowel Disease (IBD), the efficacy and the side effects of the biologics are rarely studied in the elderly population. Our aim was to evaluate retrospectively the drug sustainability, efficacy, and safety of the biologic therapies in the elderly IBD population.
Methods
Consecutive elderly (> 60 years old) IBD patients, treated with biologics (infliximab, adalimumab, vedolizumab, ustekinumab) followed at the McGill University IBD Center were included between January 2000 and 2020. The efficacy of treatment was measured using clinical and biochemical parameters. Response and remission variables were measured at three distinct time points from the start date of the biologic agent, as in 3, 6-9 and 12-18 months. Patients required a minimal follow-up period of three months. Adverse events (AE) or serious adverse events (SAE) occurring within three months of the last biologic dose were considered to be related to the biologic agent.
Results
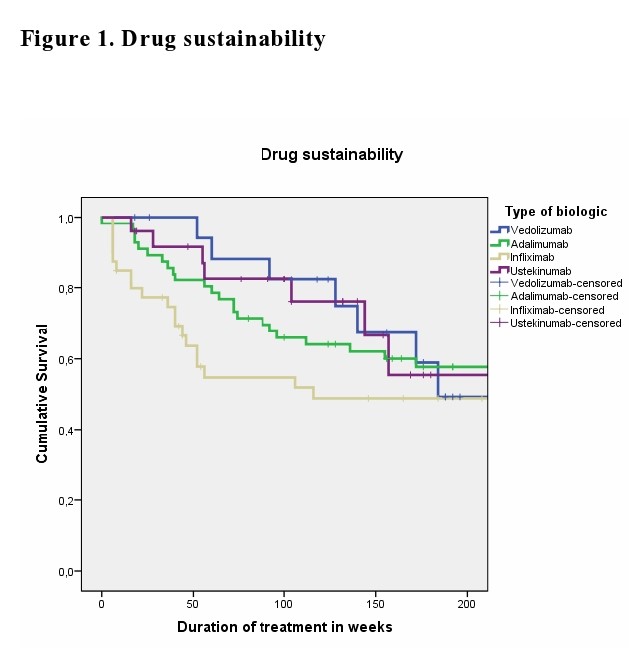
We identified a total of 147 elderly patients with IBD treated with biologic agents (109 Crohn’s disease (CD) and 38 ulcerative colitis (UC)). Disease location was predominantly ileocolonic (47.7%) in CD and pancolitis(63.2%) in UC. 47.6% of elderly IBD patients had attempted at least 1 biologic agent (Table1). The mean duration of biologic treatment was 157.5 (SD:148)weeks. Steroid exposure varied from 34% at the start of biologic therapy to 19% at 3 months, 16.3% at 6-9 months and 6.5% at 12-18 months.' The remission rates at 3, 6-9 and 12-18 month were not significant between the biologic therapies. Kaplan-Meyer analysis did not show statistical difference for drug sustainability (p=0.195)(Figure1), time to adverse event (p=0.158) (Figure2) or infection rates (p=0.973) (Figure3) between all four biologics studied. The most common AEs led to discontinuation were loss of response, infusion or injection reaction and infection.


Conclusion
Current biologic therapies were not different with regards to drug sustainability and safety in the elderly IBD population. Based on these results, we are not able to suggest a preferred sequencing order among biologicals.


