P455 Tofacitinib Therapy is Effective for Arthralgias Associated with Active Inflammatory Bowel Disease
Silfen, A.(1);Cohen, N.(1);Traboulsi, C.(1);Rodriguez, T.(1);Steinberg, J.(1);Singer, J.(1);Patel, S.(1);Cohen, R.(1);Dalal, S.(1);Micic, D.(1);Pekow, J.(1);Sakuraba, A.(1);Rubin, D.(1);
(1)University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, United States
Background
Tofacitinib is an oral Janus kinase (JAK) inhibitor used to treat ulcerative colitis (UC). It is generally well tolerated and safe with mild to no side effects. Tofacitinib is FDA-approved for use in multiple inflammatory conditions such as rheumatoid arthritis, psoriasis and polyarticular course juvenile idiopathic arthritis as well as for ulcerative colitis (UC). Patients with UC often have extra-intestinal manifestations (EIMs) with up to 20% of patients having arthropathy. There are limited data describing the effect of tofacitinib therapy on EIMs in UC. The aim of this study is to determine whether tofacitinib therapy improves arthralgia in this patient population.
Methods
This retrospective study includes consecutive patients with active UC who initiated tofacitinib at our center since 2014 and remained on the therapy for at least 8 weeks. We reviewed electronic medical records to collect demographic and clinical data. Patients diagnosed with any EIM by the treating physician were included. Improvement in EIM symptoms was determined through patient reports or physician assessment. In the UC patients, a decrease in Simple Clinical Colitis Activity Index (SCCAI) of ≥ 3 was considered response and remission as a score of ≤ 2. In the CD patients, a 3-point reduction in Harvey Bradshaw index (HBI) was considered clinical response and a score of <5 remission.
Results
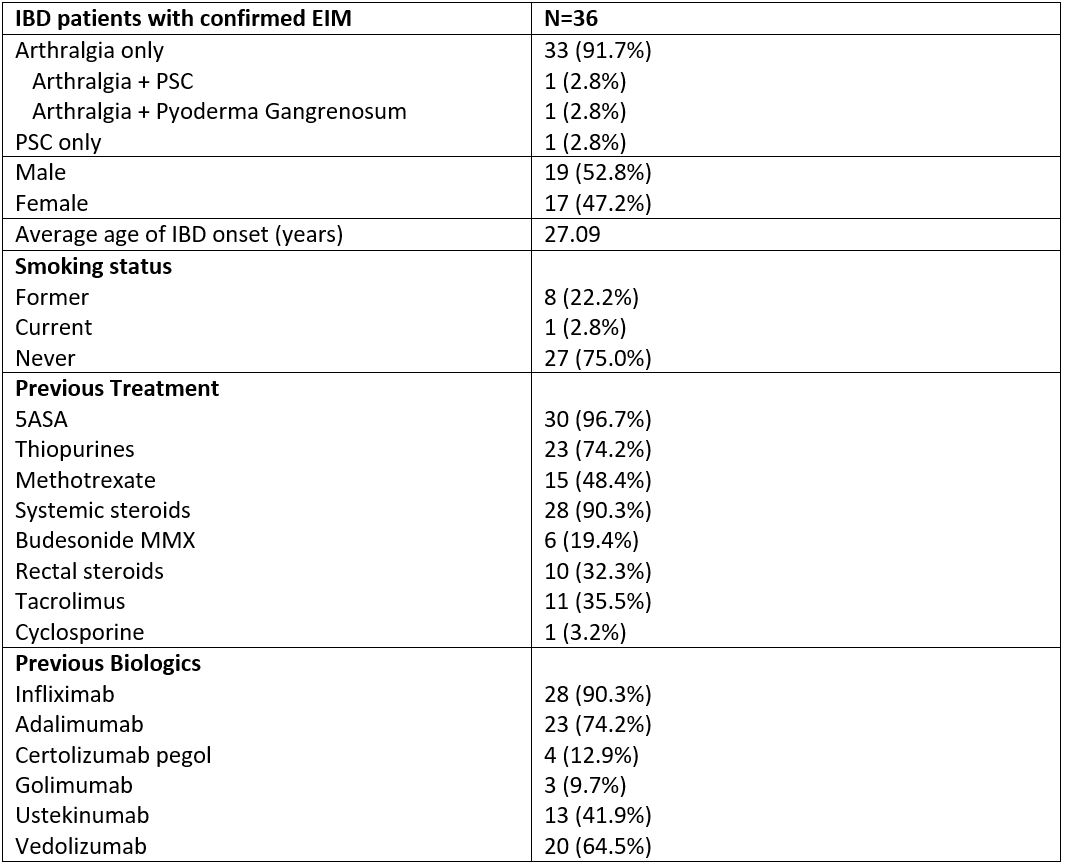
112 patients were included in this cohort, with 36 (31 UC; 5 CD) having confirmed EIMs (32.1% of total). Basic demographic and clinical data are detailed in Table 1. 35 patients had peripheral arthralgia, 2 patients had primary sclerosing cholangitis (PSC) and 1 had pyoderma gangrenosum. 5 (13.9%) patients discontinued treatment during the induction phase due to lack of response. By 24 weeks, 26 (74.2%) out of 35 patients reported improvement in arthralgias, and 14 (40%) patients reporting resolution of joint symptoms. In terms of gastrointestinal disease activity, at week 24, 17/35 (48.6%) patients achieved clinical response and 11/35(31.4%) patients achieved clinical remission. There was no treatment effect noted on the other EIMs.
Conclusion
In our real world experience, treatment refractory IBD patients with peripheral arthralgia on tofacitinib showed significant improvement in joint symptoms, and this often paralleled improvement in gastrointestinal symptoms. This supports that tofacitinib is a suitable treatment for patients with IBD and concomitant peripheral arthralgia.