P458 Safety and Potential Efficacy of Escalating Dose of Ustekinumab in Pediatric Crohn's disease (the SPEED-UP study) - A multi-center study from the paediatric IBD Porto group of ESPGHAN
Yerushalmy-Feler, A.(1);Pujol-Muncunill, G.(2);Martin-de-Carpi, J.(2);Kolho, K.L.(3);Levine, A.(4);Olbjørn, C.(5);Granot, M.(6);Bramuzzo, M.(7);Jonsson Rolandsdotter8, H.(8);Mouratidou, N.(9);Hradsky, O.(10);Scarallo, L.(11);Matar, M.(12);Magen Rimon, R.(13);Rinawi, F.(14);Shalem, T.(15);Najajra, H.(16);de Meij, T.(17);Aloi, M.(18);Velasco Rodríguez-Belvís, M.(19);Alvisi, P.(20);Schneider, A.M.(21);van Rheenen, P.(22);Navas-López, V.M.(23);Kiparissi, F.(24);Barrio, J.(25);Turner, D.(26);Cohen, S.(1);
(1)"Dana-Dwek" Children's Hospital- Tel Aviv Sourasky Medical Center and the Sackler Faculty of Medicine- Tel Aviv University, Pediatric Gastroenterology Institute, Tel Aviv, Israel;(2)Hospital Sant Joan de Déu, Department of Pediatric Gastroenterology- Hepatology and Nutrition, Barcelona, Spain;(3)Children's Hospital and University of Helsinki- Helsinki- Finland and Tampere University, Department of Paediatric Gastroenterology, Tampere, Finland;(4)Wolfson Medical Center and the Sackler Faculty of Medicine- Tel Aviv University, Pediatric Gastroenterology Unit- PIBD research center, Tel Aviv, Israel;(5)Akershus University Hospital, Department of Paediatric and Adolescent Medicine, Lørenskog, Norway;(6)Edmond and Lily Safra Children's Hospital- Sheba Medical Center, Pediatric Gastroenterology Unit, Ramat Gan, Israel;(7)Institute for Maternal and Child Health-IRCCS "Burlo Garofolo", Gastroenterology- Digestive Endoscopy and Nutrition Unit, Trieste, Italy;(8)Department of Clinical Science and Education- Karolinska Institutet, Department of Gastroenterology- Sachs' Children and Youth Hospital, Stockholm, Sweden;(9)Karolinska University Hospital, Department of Pediatric Gastroenterology- Hepatology and Nutrition, Stockholm, Sweden;(10)Charles University in Prague and Motol University Hospital, Department of Pediatrics- 2nd Faculty of Medicine, Prague, Czech Republic;(11)Meyer Children's Hospital- 50139, Gastroenterology and Nutrition Unit, Florence, Italy;(12)Schneider Children's Medical Center and the Sackler Faculty of Medicine- Tel Aviv University, Institute of Gastroenterology- Nutrition and Liver Diseases-, Tel Aviv, Israel;(13)Ruth Children's Hospital of Haifa- Rambam Medical Center- Faculty of Medicine- Technion, Pediatric Gastroenterology and Nutrition Institute, Haifa, Israel;(14)Ha'Emek Medical Centre, Paediatrics Gastroenterology Unit, Afula, Israel;(15)Shamir Medical Center, The Jecheskiel Sigi Gonczarowski Pediatric Gastroenterology Unit, Zerifin, Israel;(16)Shaare Zedek Medical Center-, The Juliet Keiden Institute of Pediatric Gastroenterology and Nutrition, Jerusalem, Israel;(17)Amsterdam University Medical Centre, Department of Pediatric Gastroenterology, Amsterdam, Netherlands Antilles;(18)Umberto I Hospital- Sapienza University of Rome, Department of Maternal and Child Health- Pediatric Gastroenterology and Liver Unit-, Rome, Italy;(19)Hospital Niño Jesús, Paediatric Gastroenterology- Hepatology and Nutrition, Madrid, Spain;(20)Maggiore Hospital, Pediatric Gastroenterology Unit- Department of Pediatrics, Bologna, Italy;(21)Paracelsus Medical University, Department of Pediatrics, Salzburg, Austria;(22)University Medical Centre Groningen Beatrix Childrens Hospital, Paediatric Gastroenterology, Groningen, Netherlands Antilles;(23)Hospital Regional Universitario de Málaga, Pediatric Gastroenterology and Nutrition Unit, Málaga, Spain;(24)Great Ormond Street Hospital for Children NHS Foundation Trust- Great Ormond Street, Department of Pediatric Gastroenterology, London, United Kingdom;(25)Hospital Universitario de Fuenlabrada, Department of Paediatrics, Madrid, Spain;(26)Shaare Zedek Medical Center and the Hebrew University of Jerusalem, The Juliet Keiden Institute of Pediatric Gastroenterology and Nutrition, Jerusalem, Israel;
Background
Ustekinumab (UST) is an effective therapy for induction and maintenance of remission in Crohn's disease (CD). Intensification of UST maintenance dosage has shown effectiveness in some adult patients, but no similar data are available in children. The aim of the study was to evaluate the effectiveness and safety of dose escalation of UST in paediatric CD.
Methods
This was a retrospective multicenter study from 25 centers affiliated to the IBD Interest and Porto groups of ESPGHAN. We included children with CD who initiated UST at a standard dosing and subsequently underwent dose escalation to intervals shorter than 8 weeks, or re-induction due to active disease. Demographic, clinical, laboratory, endoscopic and imaging data were collected at escalation and during a 12 months follow-up. Clinical remission was defined as weighted Paediatric Crohn's Disease Activity Index (wPCDAI)<12.5 and clinical response as a decline in >17.5 points. Adverse events were explicitly recorded.
Results
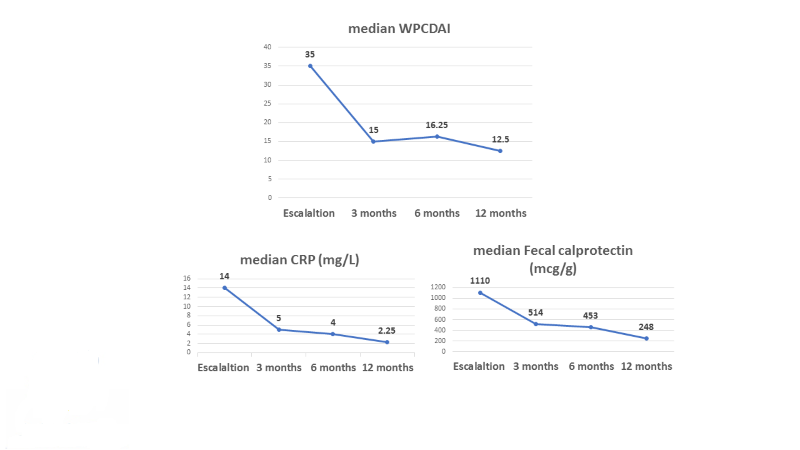
Sixty-nine children, with a median age of 15.8 (IQR 13.8-16.9) years and disease duration of 4.3 (2.9-6.3) years were included. Sixty-eight (98.6%) and 59 (86.8%) children were biologic and immunomodulators experienced, respectively. UST dose was escalated after a median of 6 (3.6-12) months of therapy. Clinical response and remission were observed in 46 (67%) and 29 (42%) children at 3 months, respectively. The strongest predictor of clinical remission was lower wPCDAI at escalation (p=0.001). The median serum levels of C-reactive protein decreased from 14 (3-28.03) to 5 (1.1-20.5) mg/L at 3 months (p=0.012), and fecal calprotectin from 1110 (499-2300) to 248 (118-1159) mcg/g at 12 months (p=0.05). Endoscopic and transmural healing were achieved in 3/19 (16%) and 2/15 (13%) patients, respectively, of those with available tests. Overall, 13 patients (19%) discontinued therapy due to active disease, at a median of 3 (3-4.5) months. No serious adverse events were reported.
Conclusion
Two-thirds of children with active CD achieved response following dose escalation of UST.