P463 Induction of endoscopic response: a network meta-analysis of induction studies comparing ontamalimab with other treatments for moderate-to-severe ulcerative colitis
A. Vickers1, A. Nag2, B. Devine3, B.E. Sands4, R. Panaccione5, L. Peyrin-Biroulet6, S. Danese7, S. Vermeire8, K.J. Gorelick9, M. Goetsch10, L. Hartley1
1RTI Health Solutions, Data Analytics and Design Strategy, Manchester, UK, 2Shire- a Takeda company, Health Economics- Outcomes Research and Epidemiology, Lexington, USA, 3School of Pharmacy, University of Washington, Seattle, USA, 4Icahn School of Medicine at Mount Sinai Medical Center, Dr Henry D. Janowitz Division of Gastroenterology, New York, USA, 5Division of Gastroenterology, University of Calgary, Calgary, Canada, 6Department of Gastroenterology, University Hospital of Nancy-Brabois, Vandœuvre-lès-Nancy, France, 7IBD Center, Humanitas University, Milan, Italy, 8Department of Gastroenterology, University Hospital Leuven, Leuven, Belgium, 9Zymo Consulting Group, Clinical Development, Newtown Square, USA, 10Shire- a Takeda Company, Clinical Development, Zug, Switzerland
Background
Clinicians, patients, payers and policymakers require relevant, high-quality evidence to support decision-making regarding the treatment of ulcerative colitis (UC). In the absence of head-to-head trials, network meta-analysis (NMA) can be used to compare treatments. We conducted an NMA to compare the efficacy of ontamalimab (anti-MAdCAM-1) using its phase 2 data, with all biologics and novel small molecules for which induction study data on endoscopic response were available.
Methods
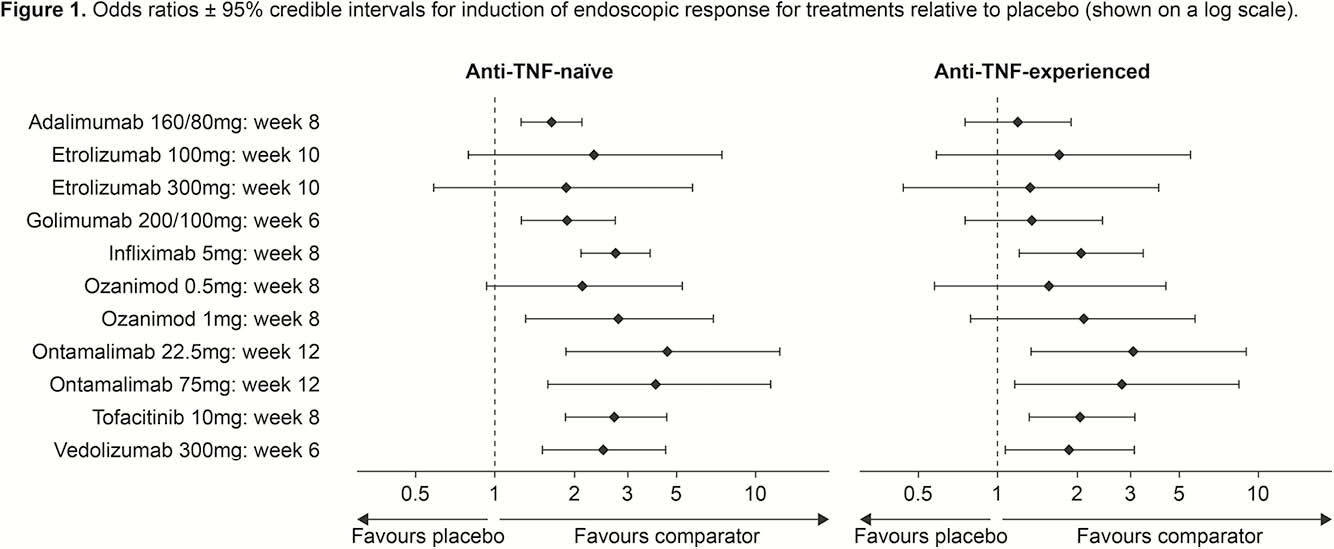
A systematic literature review was conducted in November 2017 to identify published randomised controlled trials of induction treatment in patients with moderate-to-severe UC. An NMA of the identified studies was performed using random-effects models and methods based on NICE guidance. Odds ratios and 95% credible intervals were calculated to describe the relative differences between treatments and placebo in terms of efficacy in inducing endoscopic response. Results were examined by anti-TNF status (naïve vs. experienced).
Results
In total, 15 phase 2 and phase 3 induction studies of the following agents were available and included: adalimumab (160/80mg), etrolizumab (100mg and 300mg), golimumab (200/100mg), infliximab (5mg), ontamalimab (22.5mg and 75mg), ozanimod (0.5mg and 1mg), tofacitinib (10mg) and vedolizumab (300mg). The definition of endoscopic response (improvement) in all trials was a Mayo endoscopic subscore of ≤1. Homogeneity between studies was good, enabling pooling of results. Figure 1 shows odds ratios for induction of endoscopic response with treatments relative to placebo in anti-TNF-naïve and -experienced patients. All treatments performed significantly better than placebo in anti-TNF-naïve patients, with the exception of both doses of etrolizumab and ozanimod 0.5 mg. Significant differences between some treatments were observed; specifically, ontamalimab 22.5 mg (
Conclusion
This study suggests that ontamalimab, infliximab and tofacitinib could be superior to adalimumab in inducing endoscopic healing, although it was conducted before any large-scale head-to-head trials of these drugs. Furthermore, large variances due to differing endpoint timings, the combination of phase 2 and phase 3 data, and lack of control for placebo response rates preclude firm conclusions being drawn.


