P465 One year endoscopic and histologic outcomes to tofacitinib therapy in refractory ulcerative colitis
Verstockt., B.(1,2);Aden, K.(3,4);Alsoud, D.(2);Outtier, A.(1);Sabino, J.(1,2);Rosenstiel, P.(3,4);Vermeire, S.(1,2);De Hertogh, G.(5);Schreiber, S.(3,4);Ferrante, M.(1,2);
(1)University Hospitals Leuven, Dpt. Gastroenterology and Hepatology, Leuven, Belgium;(2)KU Leuven, Dpt. Chronic Diseases- Metabolism and Ageing- TARGID-IBD, Leuven, Belgium;(3)Christian-Albrechts-University and University Hospital Schleswig-Holstein, Institute of Clinical Molecular Biology, Kiel, Germany;(4)Christian-Albrechts-University and University Hospital Schleswig-Holstein, Department of Internal Medicine I, Kiel, Germany;(5)University Hospitals Leuven, Laboratory of Morphology and Molecular Pathology, Leuven, Belgium
Background
Long-term real-life data on the efficacy of the Janus kinase inhibitor tofacitinib in moderate-to-severe ulcerative colitis (UC) are limited. We here report efficacy of tofacitinib in refractory UC patients with an emphasis on endoscopic and histologic outcome.
Methods
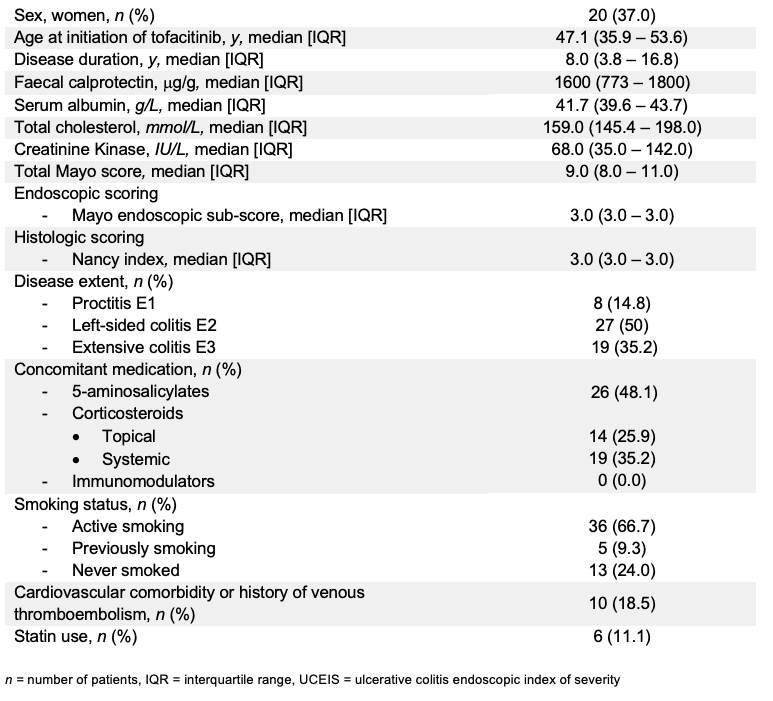
Fifty-four consecutive UC patients (Mayo endoscopic sub-score ≥2) initiating tofacitinib in 2 tertiary IBD referral centres were prospectively included (Table 1). Almost all were refractory to both anti-TNF (96.4%) and vedolizumab (92.7%) and received tofacitinib in standard dosage. Steroid-free clinical remission was defined as partial Mayo score of ≤2 with no single sub-score >1 and without any steroid exposure at assessment. Biological remission was defined as faecal calprotectin <250mcg/g, endoscopic remission as Mayo endoscopic sub-score of 0, endoscopic improvement as Mayo endoscopic sub-score of ≤1, and histologic remission as Nancy histology index of 0. A combination of endoscopic and histologic remission was referred as mucosal healing. All outcomes were assessed at year 1. Non-responder imputation and last observation carried forward analysis were applied.
Table 1
Results
Patients were followed for a median [IQR] of 91.7 [67.2-102.4] weeks, with an exposure to tofacitinib of 23.9 [14.6-51.6] weeks. By year one, 31.5% of patients were in steroid-free clinical remission (Figure 1). Biological remission was observed in 26.9% of patients. Endoscopic improvement, endoscopic remission, histologic remission and mucosal healing, were observed in 31.5%, 24.1%, 22.0% and 18.0% of patients, respectively. Multivariate analysis identified baseline Mayo endoscopic sub score (OR 0.03, p=0.03); endoscopic improvement by week 16 (OR 36.5, p=0.008) and disease extent (OR 0.11, p=0.05) as independent predictors for endoscopic remission at year 1. Patients with left-sided colitis or proctitis experienced higher endoscopic remission rates (33.3% and 25.0%) then patients with extensive colitis (10.5%, p=0.09; p=0.6). Ultimately, 61.1% of all patients discontinued tofacitinib therapy after a median of 15.9 [9.2-24.5] weeks, due to primary non-response (n=25), loss-of-response (n=6) or (serious) adverse events (n=2). Thirteen patients (24.1%) required colectomy. During follow-up, no venous thrombo-embolisms or cancers were observed. One patient had to be admitted at ICU due to several life-threatening opportunistic infections.
Figure 1
Conclusion
In this highly refractory cohort of UC patients, tofacitinib induced and maintained endoscopic and histologic remission in up to one quarter of patients. UC patients with moderate left-sided colitis and proctitis had a numeric higher likelihood for a sustained effect than patients with extensive colitis.


