P468 Evaluation of work productivity and activity impairment in moderate-to-severe ulcerative colitis participants treated with ozanimod in the phase 3 True North study
Floden, L.(1);Pham, T.P.(2);Kumar, J.(3);Becker, B.(3);Shaw, J.W.(3);Tencer, T.(3);
(1)Clinical Outcomes Solutions, Biostatistics, Chicago, United States;(2)Clinical Outcomes Solutions, Research, Chicago, United States;(3)Bristol Myers Squibb, Department of Immunology and Fibrosis Development, Princeton, United States;
Background
Ulcerative Colitis (UC) is an autoimmune disease which negatively impacts patients’ quality of life including work performance. This study examined the effect of ozanimod on work productivity and activity impairment (WPAI) in participants with moderate-to-severe UC in the phase 3 True North study.
Methods
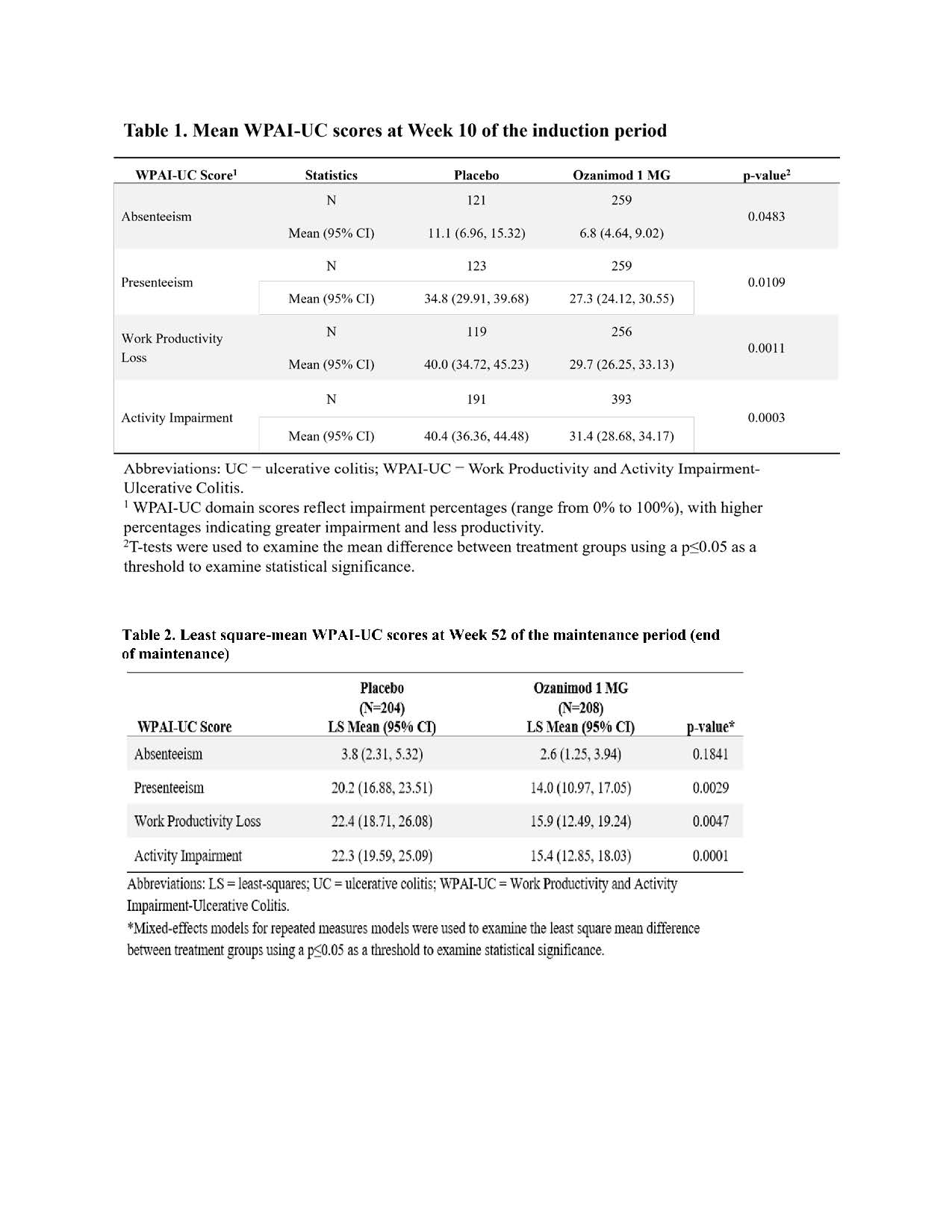
Participants who received 1 mg QD ozanimod during a 10-week Induction Period (IP) and were clinical responders from both placebo-controlled and open-label cohorts were randomised to ozanimod (n=230) or placebo (n=227) for a 42-week Maintenance Period (MP). Work productivity was measured with the WPAI-UC patient-reported outcome instrument that assesses absenteeism, presenteeism, work productivity loss, and activity impairment due to UC over the previous week. The WPAI-UC was administered at Weeks 10, 28, 40, and 52. Differences in WPAI-UC domain scores at the end of the IP were evaluated using t tests. Mixed-effects models for repeated measures (MMRMs) were fit to estimate least-squares mean WPAI-UC scores adjusted for treatment, time (categorical), clinical variables, and corticosteroid use at Week 10. MMRMs were also applied to evaluate differences in WPAI domain scores between groups defined by clinical response, clinical remission, and endoscopy score. Generalised estimating equation (GEE) models were applied to estimate the odds of improving or remaining stable (using a ≥7% change threshold) on WPAI-UC domains for those on ozanimod versus placebo. Missing values were multiply imputed using a placebo-based imputation model; estimates were combined using Rubin’s rules.
Results
At the end of the IP, the ozanimod group had significantly lower impairment of work productivity and activity (WPA) than the placebo group (Tab 1). During MP, the ozanimod group reported significantly lower levels of impairment for presenteeism (14.0% vs 20.2%; P=.003), work productivity loss (15.9% vs. 22.4%; P=.005), and activity impairment (15.4% vs 22.3%; P=.0001) compared to the placebo group (Tab 2). At Week 52, participants with improved endoscopy score, in clinical remission, or in clinical response had significantly lower impairment in all WPAI-UC domains (Tab 3). Participants in the ozanimod group showed increased odds of improving or remaining stable compared to those in the placebo group for all WPAI-UC domains (Fig 1).
Conclusion
Participants in the ozanimod arm had significantly better work productivity at the end of induction. Among participants who received ozanimod and achieved response in IP, ozanimod maintenance treatment was associated with improvements in WPA. These improvements were greater among participants who achieved clinical response or remission or improved endoscopic score.